Researchers uncover how protein receptors on cells switch on and off for growth and health
By Susan S. Lang
Cornell researchers have provided new insight into the molecular mechanism underlying an essential cellular system. They have discovered how receptors on cell surfaces turn off signals from the cell's environment, a function that is vital for cell functions such as growth, division and death.
The findings, reported in the Jan. 9 issue of the journal Cell (136:1), have important implications for better understanding cancer, AIDS, neurodegenerative disorders and other illnesses, because such diseases can result when receptors go awry by failing to turn off, a function known as down-regulation.
A wide variety of receptors, which are composed of proteins, at the cell surface control interactions between cells and their environment. The machinery responsible for down-regulating the cell surface receptors is called ESCRT (for endosomal sorting complex required for transport). This machinery packages receptors into temporary transport structures called multivesicular bodies (MVBs) that control the down-regulation and, ultimately, the degradation of the receptors.
Using baker's yeast as a model genetic system, researchers working with Scott Emr, director of Cornell's Weill Institute for Cell and Molecular Biology and professor of molecular biology and genetics, have discovered how the ESCRT machinery directs the formation of the MVB transport structure.
"The ESCRT machinery catalyzes a transport reaction that is topologically opposite to most other intercellular transport reactions in the cell," said Emr.
In other words, rather than forming small membrane vesicles that bud into the cytoplasm of the cell, like those that deliver secreted insulin to the cell surface, the MVB membrane compartment contains membrane vesicles within it that form by budding out of the cytoplasm and into the interior of the MVB. The ESCRT machinery, Emr's team found, deforms the cell membrane to generate these internal vesicles.
The team's findings also suggest that the machinery assembles by forming a ringlike filament that encircles receptors that need to be packaged in the MVBs.
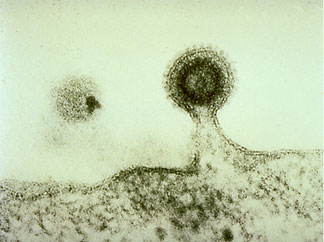
"This ESCRT ring also serves as a scaffold to direct the topology of membrane deformation," Emr said. The ESCRT ring helps viruses like HIV deform the membrane and thereby bud out of the cell, forming the free virus that can then go on to infect healthy cells in AIDS patients.
"Diverse biological processes -- MVB formation, cell division and viral budding -- share the same budding topology," Emr explained. "Interestingly, these seemingly unrelated processes all require the function of the ESCRT machinery."
"In addition, Suraj Sakena and David Teis, postdoctoral fellows in my lab, have used an elegant fluorescence-based approach to define the sequence of this process, as well as the assembly and disassembly of the ESCRT machinery," Emr said.
"Considering the role of the ESCRT proteins in devastating human diseases like AIDS (see picture), a detailed biochemical understanding of how ESCRT proteins function will certainly enhance future biotechnological developments that could result in the discovery of new drugs for the treatment of these diseases," Emr said.
The research was funded in part by the National Institutes of Health, the Robert A. Welch Foundation and the Weill Institute for Cell and Molecular Biology.
Media Contact
Get Cornell news delivered right to your inbox.
Subscribe