Worm sugarcoats bacterial toxins to stave off death
By Karen Kindle
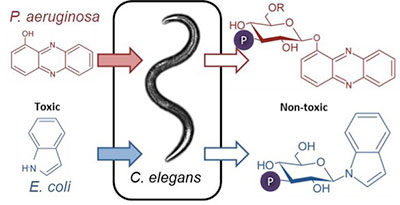
Pathogenic bacteria kill their animal or plant hosts through the production of toxic molecules. But how do animals and plants defend themselves against these toxins? Researchers from the Boyce Thompson Institute for Plant Research (BTI), located at Cornell University, and the University of Florida (UF) have found that the tiny nematode C. elegans chemically disables bacterial toxins: The worms attach a sugar molecule (glucose), which renders toxic bacterial molecules harmless. The paper was published in the Nov. 19 issue of ACS Chemical Biology.
Frank Schroeder (BTI), Arthur Edison (UF) and colleagues examined how C. elegans defends itself against two unrelated bacterial toxins released by P. aeruginosa and E. coli, both of which can kill the worm. They found that C. elegans adds a sugar residue to an oxygen atom in the P. aeruginosa toxin and to the nitrogen in the E. coli toxin. The worms go on to add a phosphate, which results in chemicals far less toxic than the original bacterial compounds.
“Worms employ detoxification mechanisms very similar to those we know from humans, though they likely use a different family of enzymes,” Schroeder said. Elucidation of the enzymes the worms use to modify toxins could lead to the development of detoxification inhibitors, which could improve the efficacy of existing drugs for parasitic roundworm infections in humans, livestock and plants.
Schroeder’s laboratory works on the structural identification of small molecules and their biological functions in nematodes. His group focuses primarily on C. elegans, a harmless, nonparasitic worm used as a model for disease-causing parasitic nematodes. In addition, C. elegans research has provided major insights in evolutionarily conserved aspects of human biology; for example, the mechanisms underlying diabetes, aging and neurological diseases.
This research was funded by the National Institutes of Health.
Karen Kindle is principal liaison for technology marketing and licensing at BTI.
Media Contact
Get Cornell news delivered right to your inbox.
Subscribe