Exercise could reduce bone tumor growth
By Anne Ju
Weight-bearing exercise, often prescribed to combat bone loss, might have anti-cancer effects. Cornell biomedical researchers report that mechanical stimulation of cancerous bone, in making bone stronger, seems to make tumors weaker.
The study, which involved both animal and in vitro models, showed that causing bone to bear moderate, repeated weight – akin to an exercise regimen – led to inhibited growth of bone tumors composed of metastasized breast cancer cells. The researchers think that such mechanical stimulation might reduce the expression of genes that interfere with normal, healthy bone functioning.
Published online May 3 by the Journal of Bone and Mineral Research, the study was led by first author and postdoctoral researcher Maureen Lynch and senior author Claudia Fischbach-Teschl, associate professor of biomedical engineering.
Breast cancer often metastasizes to bone, which then deteriorates because a tumor disturbs the normal process of bone remodeling. The subsequent bone loss, which is caused by too much bone breakdown known as osteolysis, helps feed the tumor, because the bone is a depot of factors that promote tumor growth. Degraded bone releases these stored factors, and the tumor grows.
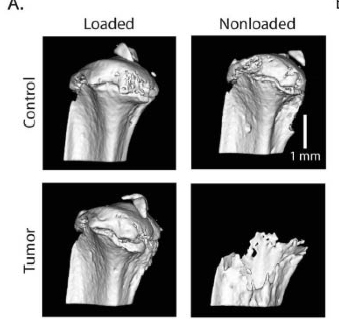
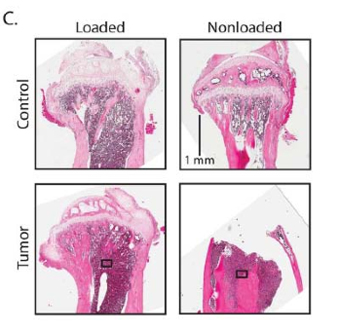
The researchers used a bone “loading model” to show that tumors respond to mechanical stimulation. The loading model originated in the lab of paper co-author Marjolein van der Meulen, the Swanson Professor of Biomedical Engineering at Cornell, who studies biological mechanics particularly in bone.
The experiments showed that loading of a mouse tibia injected with malignant breast cancer cells – recreating the metastatic condition – kept the tumor from growing and increased bone mass. Doing nothing allowed the tumor to proliferate and the bone to degrade.
“If you think about typical cancer treatment, like chemotherapies, they are targeting the cancer cells,” Lynch said. “So we needed to figure out if loading is affecting the tumor cells in addition to the bone cells, or if this is some kind of indirect effect. We found a little bit of both.”
To control microenvironmental factors, the researchers did other experiments with a 3-D tumor model designed in Fischbach-Teschl’s lab. They injected tumor cells into a porous scaffold that looks like an aspirin pill, then loaded the cells by using a piston to squeeze the scaffold. They examined how different genes characteristic of tumor cells were expressed as the loading occurred.
Genes involved in cancer were largely unaffected, except one, called Runx2, a transcription factor that regulates gene expression and production of other proteins. This, in turn, affects what bone cells are doing.
In a cancer cell, the researchers have theorized, Runx2 directs the cells to secrete proteins that stimulate osteolysis, too much of which leads to bone loss. Mechanical loading inhibits expression of Runx2, and they think that’s why proteins that affect osteolysis are also reduced. Further experimentation to confirm this theory is needed.
The insights from this paper could lead not only to drug therapies but also to more targeted exercise regimens for cancer patients, Fischbach-Teschl said.
“Specifically, physically mediated mechanisms in bone might be contributing to bone loss in cancer patients,” she said. “So harnessing those things better is what could really help patients.”
The paper is titled “In Vivo Tibial Compression Decreases Osteolysis and Tumor Formation in Human Metastatic Breast Cancer Model.” The work was supported by the National Institutes of Health and The Hartwell Foundation.
Media Contact
Get Cornell news delivered right to your inbox.
Subscribe