Gene prevents buildup of misfolded cell proteins
By Krishna Ramanujan
Much like how a snowplow is needed to clear streets of heavy snow, cells employ a set of genes to clear away misfolded proteins, to prevent them from accumulating and destroying the cell.
For the first time, Cornell researchers have demonstrated how a gene called SEL1L plays a critical role in clearing away misfolded proteins. Complications from misfolded proteins lead to cell death and underlie numerous diseases, including Type 1 diabetes and cystic fibrosis.
SEL1L works within one of several known complexes, known as the endoplasmic reticulum-associated degradation (ERAD), which survey and detect misfolded proteins, grab them and target them for degradation before they accumulate and cause havoc. The endoplasmic reticulum is the cell’s protein-making machinery, and each ERAD complex is responsible for preventing a subset of these misfolded proteins.
“Physiologically, we know almost nothing about the significance of individual ERAD complexes and how they function together in vivo,” said Ling Qi, Cornell associate professor of molecular and biochemical nutrition and senior author of a paper published online Jan. 22 in the Proceedings of the National Academy of Sciences. “Our study tells us that SEL1L is like the engine of a snowplow; without it, we have no ability to clear the snow in the streets, and cells cannot prevent misfolded proteins from accumulating,” said Qi.
“Previous research has shown that the SEL1L gene plays a critical role in managing misfolded proteins in yeast, but studying the gene in mammals proved difficult until now,” said Qiaoming Long, Cornell assistant professor of animal science and co-senior author on the paper, who has been studying the gene since 2005.
To determine the function of a gene, researchers develop mice without a gene of interest, raise them and look for deficiencies in the mice to determine that gene’s role. But mice without the SEL1L gene died as embryos. As a result, Qi teamed up with Long to develop mice that were born with the gene, but after birth, the gene could be silenced with injections of a drug called tamoxifen. Without the SEL1L gene, the researchers showed that the mice developed exocrine pancreatic insufficiency, a disease found in humans, dogs and cats, where the animals fail to digest and absorb food, become severely malnourished and develop shriveled pancreases.
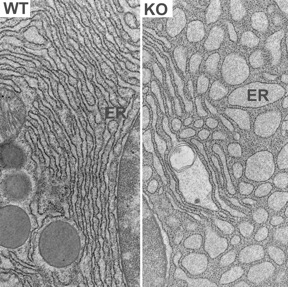
“When we looked at the cells in the pancreas, we were amazed that the endoplasmic reticulum becomes dilated and fragmented; cells are clearly in the stressed state,” said Shengyi Sun, a graduate student and the paper’s co-first author, along with Guojun Shi, a postdoctoral associate, both of whom work in Qi’s lab.
In future work, Qi, Long and colleagues will look at the role that SEL1L plays in other tissue types and diseases, such as fat cells in obesity and Type 2 diabetes, intestinal cells in inflammatory bowel disease, and more. Another area of interest will include identifying proteins that this ERAD complex tags and degrades.
“Accumulation of misfolded proteins and collapse of the endoplasmic reticulum is a characteristic of disease pathogenesis,” said Qi. “Now we can target this complex” with therapies. “These findings will have a profound impact” on future treatments, he said.
Other researchers on the paper were Cornell’s Adam Francisco, Gerald Duhamel, Kenneth Simpson, Yewei Ji, Xiaojing Liu and Jason Locasale; Xuemei Han and John Yates of the Scripps Research Institute; and Nuno Mendonca and Sander Kersten of Wageningen University in the Netherlands.
The study was funded by the National Institutes of Health, the Netherlands Nutrigenomics Centre, the American Diabetes Association, Cornell Vertebrate Functional Genomics Center, National Science Foundation of China and Howard Hughes Medical Institute.
Media Contact
Get Cornell news delivered right to your inbox.
Subscribe