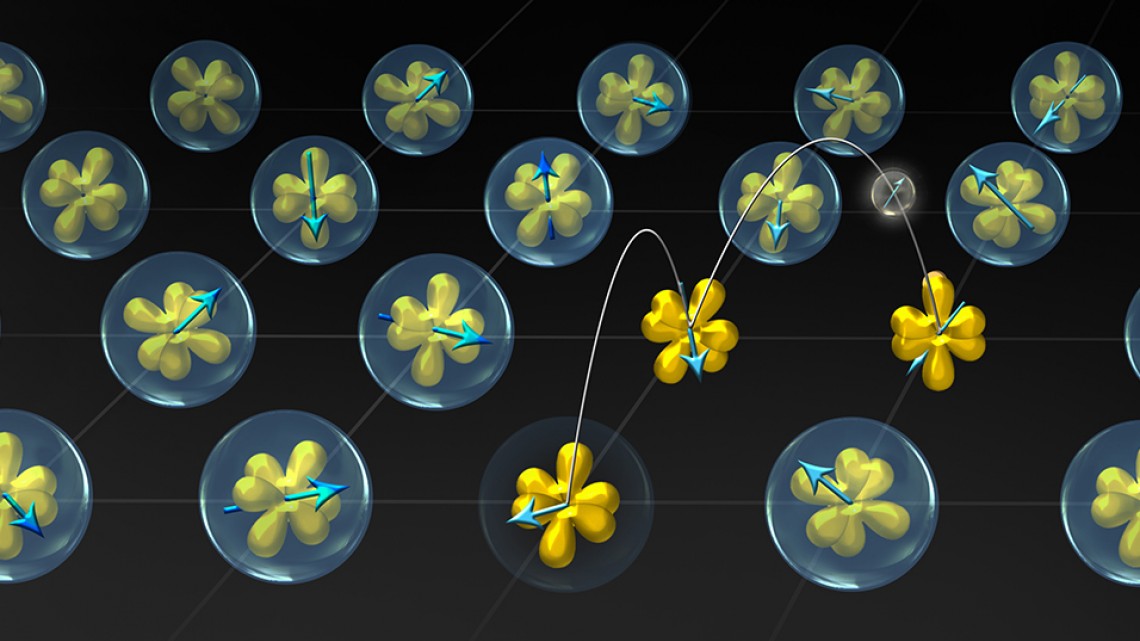
Illustration of ytterbium (Yb) atoms in YbAl3, where electrons transform from localized states (bubbles surrounding the yellow orbitals) to itinerant states (hopping amongst orbitals), as a function of temperature.
Where did those electrons go? Decades-old mystery solved
By Tom Fleischman
The concept of “valence” – the ability of a particular atom to combine with other atoms by exchanging electrons – is one of the cornerstones of modern chemistry and solid-state physics.
Valence controls crucial properties of molecules and materials, including their bonding, crystal structure, and electronic and magnetic properties.
Four decades ago, a class of materials called “mixed valence” compounds was discovered. Many of these compounds contain elements near the bottom of the periodic table, so-called “rare-earth” elements, whose valence was discovered to vary with changes in temperature in some cases. Materials comprising these elements can display unusual properties, such as exotic superconductivity and unusual magnetism.
But there’s been an unsolved mystery associated with mixed valence compounds: When the valence state of an element in these compounds changes with increased temperature, the number of electrons associated with that element decreases, as well. But just where do those electrons go?
Using a combination of state-of-the-art tools, including X-ray measurements at the Cornell High Energy Synchrotron Source (CHESS), a group led by Kyle Shen, professor of physics, and Darrell Schlom, the Herbert Fisk Johnson Professor of Industrial Chemistry in the Department of Materials Science and Engineering, have come up with the answer.
Their work is detailed in a paper, “Lifshitz transition from valence fluctuations in YbAl3,” published Oct. 11 in Nature Communications. The lead author is Shouvik Chatterjee, Ph.D. ’16, formerly of Shen’s research group and now a postdoctoral researcher at the University of California, Santa Barbara.
To address this mystery, Chatterjee synthesized thin films of the mixed-valence compound of ytterbium – whose valence changes with temperature – and aluminum, using a process called molecular beam epitaxy, a specialty of the Schlom lab. The group then employed angle-resolved photoemission spectroscopy (ARPES) to investigate the distribution of electrons as a function of temperature to track where the missing electrons went.
“Typically for any material, you change the temperature and you measure the number of electrons in a given orbital, and it always stays the same,” Shen said. “But people found that in some of these materials, like the particular compound we studied, that number changed, but those missing electrons have to go somewhere.”
It turns out that when the compound is heated, the electrons lost from the ytterbium atom form their own “cloud,” of sorts, outside of the atom. When the compound is cooled, the electrons return to the ytterbium atoms.
“You can think of it as two glasses that contain some water,” Shen said, “and you’re pouring back and forth from one to the other, but the total amount of water in both glasses remains fixed.”
This phenomenon was first proposed by 20th-century Russian physicist Evgeny Lifshitz, but an answer to the electron mystery hadn’t been proposed until now.
Said Chatterjee: “These findings point toward the importance of valence changes in these material systems. By changing the arrangement of mobile electrons, they can dramatically influence novel physical properties that can emerge.”
“This places our understanding of these materials on a better footing,” Shen said.
Other contributors included Ken Finkelstein, senior staff scientist at CHESS; and doctoral students Jacob Ruf and Haofei Wei of the Shen Group.
This work was supported by grants from the National Science Foundation to CHESS and the Cornell Center for Materials Research. Some of the work was done at the Cornell NanoScale Science and Technology Facility, also supported by the NSF. Other support came from the Gordon and Betty Moore Foundation and the Air Force Office of Scientific Research.
Media Contact
Get Cornell news delivered right to your inbox.
Subscribe