Laser microscopy technique settles long debate about brain chemistry, could aid studies of Alzheimer's and stroke damage, Cornell biophysicists report
By Roger Segelken
A laser-based microscopy technique may have settled a long-standing debate among neuroscientists about how brain cells process energy -- while explaining what's really happening in PET (positron emission tomography) imaging and offering a better way to observe the damage that strokes and neurodegenerative diseases, such as Alzheimer's, wreak on brain cells.
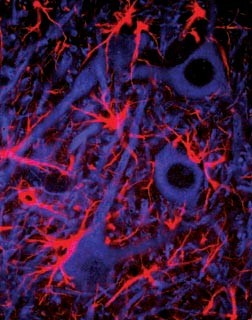
Multi-photon microscopy scans by Cornell University biophysicists of living brain tissue, as reported in the latest issue of Science (July 2, 2004), reveal exactly how and when neurons (the cells that do the thinking) and astrocytes (the starburst-shaped glial cells that service neurons) interact to burn oxygen and glucose, after astrocytes make lactate from glucose in the bloodstream, to meet the extraordinary energy demands of the brain.
Based on imaging of two different energy states of NADH (nicotinamide adenine dinucleotide, a coenzyme involved in brain-cell metabolism), the Cornell biophysicists say they have both confirmed and redefined the controversial "astrocyte-neuron lactate shuttle" hypothesis for brain energy metabolism.
"Over the past decade scientists have passionately debated whether the activated brain burns glucose completely to water or incompletely to lactate," said Karl A. Kasischke, M.D., lead author of the Science paper titled "Neural Activity Triggers Neuronal Oxidative Metabolism Followed by Astrocytic Glycolysis." "Our results unify existing contradictory opinions and should be a win-win situation for both factions," said Kasischke, who is a research associate in the Developmental Resource for Biophysical Imaging and Opto-electronics (DRBIO) laboratory headed by Watt W. Webb. Webb, Cornell's S.B. Eckert Professor in Engineering and a co-inventor of multiphoton microscopy who also is an author on the Science paper, explains: "Multiphoton microscopy imaging of intrinsic fluorescence in NADH shows that early oxidative metabolism in neurons is eventually sustained -- after about 10 seconds -- by late activation of the astrocyte-neuron lactate shuttle. Neurons, even at rest, are always burning glucose and they continue to do so when a signal begins to pass through the neurons. Then the astrocytes 'kick in' to provide lactate fuel that they have converted from glucose."
If that seems like a fine point, it is one that escaped scientists who developed PET and fMRI (functional magnetic resonance imaging) scanning technologies, as well as the medical personnel who use those diagnostic tools everyday without knowing exactly what the images represent. PET and fMRI are not recording neural activity directly, but rather surrogates for activity -- changes in blood flow (in the case of PET) and blood oxygenation (fMRI) -- Kasischke explained. He compares PET and fMRI scans to pictures from cameras with slow shutter speeds and wide-angle lenses, producing a broad view over a relatively lengthy time span. A very different picture emerges from multiphoton microscopy, which can record millisecond changes in microscopic detail. The ultra-fast microscopic technique can image individual nerve cells and even their finest extensions, where important steps in brain-cell metabolism take place.
Multiphoton microscopy is a patented technology that produces high-resolution, three-dimensional images of tissues -- in the central nervous system, for example -- with minimal damage to living cells. The procedure begins when extremely short, intense pulses of laser light are directed at cells below the surface. The rapid-fire nature of multiphoton microscopy increases the probability that two or three photons will interact with individual biological molecules at the same time, combining their energies. The cumulative effect is the equivalent of delivering one photon with twice the energy (half the wavelength, in the case of two-photon excitation) or three times the energy (one-third the wavelength in three-photon excitation) to illuminate the smallest details. As a scanning laser microscope moves the focused beam of pulsed photons across a sample at a precise depth (plane of focus); cells above or below the plane are not affected. When repeated scans at different focal planes are "stacked" by computer processing, a brilliant, three-dimensional picture emerges. And when a series of scans are "pasted" together, a movie of split-second changes at the cellular or subcellular level results.
Kasischke has been using multiphoton microscopy to observe brain cell death as it happens in strokes, a process he has helped illuminate over the past 10 years. Webb expects the technology to reveal more about malfunctioning brain cells in neurodegenerative diseases, such as Alzheimer's and Parkinson's, now that the chemical nature of brain metabolism is better understood. Other collaborators in the study are Harshad D. Vishwasrao and Warren R. Zipfel, senior research associates in Cornell's School of Applied and Engineering Physics; and Patricia J. Fisher, senior research associate in biomedical sciences at Cornell's College of Veterinary Medicine. The experiments and analysis were funded, in part, by grants from Deutsche Forschungsgemeinschaft (the German Research Foundation) and the U.S. National Institutes of Health.
Media Contact
Get Cornell news delivered right to your inbox.
Subscribe