Twice-engineered bacteria scavenge heavy-metal pollutants and hold them for recycling, Cornell biotechnologists report
By Roger Segelken
ANAHEIM, CALIF. -- Biotechnologists at Cornell University have engineered a strain of bacteria with two environment-saving abilities: To soak up heavy-metal pollutants, such as mercury, and then to sequester them for recycling.
Now the researchers are ready to begin field trials of a system that should reduce water and soil contamination to the parts-per-trillion level.
This bioremediation process kills the bacteria, and that's a good thing, Cornell chemist David B. Wilson and graduate students Shaolin Chen and Zhiqui Hao reported today (March 24) at the national meeting of the American Chemical Society at the Anaheim Hilton.
Focusing first on mercury, one of the most toxic heavy metals in the environment, the Cornell researchers engineered Escherichia coli (E. coli) bacteria both with genes from another bacterium -- to seek and transport mercury through E. coli cell membranes -- as well as genes from yeast -- to accumulate mercury inside each E. coli cell. The same strategy, they believe, can be used to re-engineer E. coli specifically to scavenge metals such as cadmium, zinc, nickel or manganese, pointing to these potential benefits that other processes do not offer:
- Cornell's recombinant bacteria take over where other processes, such as ion-exchange resins or bioabsorbents, leave off by removing heavy metals from very dilute solutions and reducing the contamination to the lowest detectable level -- less than 10 parts per trillion.
- The scavenging bacteria function efficiently in a variety of chemical environments, including solutions with a pH anywhere between 3 and 11 (most other processes need a pH between 6 and 8) and in the presence of remediation chemicals, such as chelation agents, and other metallic contaminants.
- No additional energy source, such as glucose, is needed to power the process; endogenous energy in the bacterial cells is sufficient.
- The bioengineered cell membrane targets and transports only a specific metal, such as mercury, and does not begin work until the transport genes are switched on by IPTG induction.
- More than 25 percent of the bacteria's protein (glutathione S-transferase yeast metallothionein fusion protein) is dedicated to binding the heavy metal.
- The scavenged metal is safely contained inside the bacterial membrane until released by high-temperature incineration. Then the pure metal can be recovered and recycled.
- When couples struggle with juggling careers and families, wives typically scale back. Only 15 percent of men in the sample report priority being given to their wives' careers, compared with 50 percent of women who say their husbands' careers have priority.
- Factors related to workers expecting to change jobs include workload, lack of control over scheduling and lack of opportunities for flextime and telecommuting.
"Unfortunately for the bacteria, this process kills them but that's not a bad deal for the rest of us," Wilson commented. "We don't have to worry about these organisms escaping after their work is done." Gene escape is not a major issue with the metal-scavenging bacteria, he said, because any organisms that receive the genes to transport and accumulate deadly metals do not survive and reproduce in the natural environment.
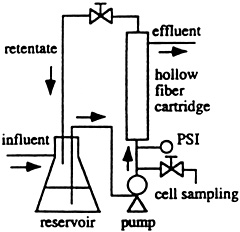
To date, the process has been tested successfully in laboratory-scale bioreactors with hollow-fiber filters holding billions of bacterial cells while water-borne mercury is pumped through the bioreactor. Scaling up the process, Wilson envisions bioremediation using mobile bioreactors working at sites with water or soil contamination, or with factory-based bioreactors to treat effluent from industrial processes.
The bacterial process would not be a cost-effective primary treatment for high concentrations of mercury or other pollutants, Wilson said "but in dilute solutions we can get every last bit of mercury." That is important, he added, "because mercury, even in low concentrations, is turning out to be more toxic than we thought." His laboratory is close to developing bioengineered bacteria to scavenge cadmium, and next will work on a similar system for cleaning up nickel contamination.
At Cornell. Wilson is a professor of biochemistry, molecular and cell biology as well as director of the Institute for Comparative and Environmental Toxicology. Development of the metal-scavenging bacteria was supported, in part, by a Superfund Basic Research and Education Program grant from the U.S. Environmental Protection Agency.
Media Contact
Get Cornell news delivered right to your inbox.
Subscribe