Protein that culls damaged eggs identified, infertility reversed
By Krishna Ramanujan
A new discovery by Cornell researchers may lead to therapies that allow women who are made infertile by radiation or chemotherapy treatments to have children.
Cornell researchers have identified a protein that tags eggs with damaged DNA and initiates a process to rid the female body of bad eggs during meiosis, when cells recombine DNA and divide to make sperm and eggs, according to a study published Jan. 31 in Science.
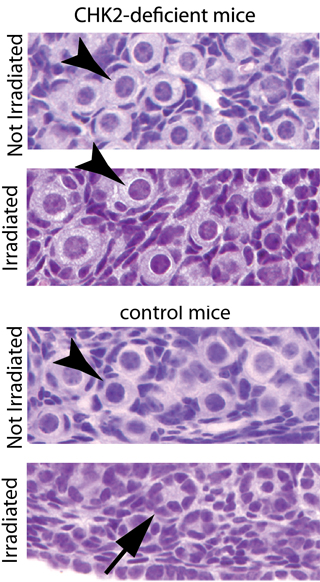
The protein, called checkpoint kinase 2 (Chk2), is essential for culling mouse oocytes – immature egg cells – with unrepaired breaks in chromosomes. Such damage may occur during meiosis or due to radiation, for example. The protein’s actions are time sensitive, so if DNA damage cannot be repaired within a certain time frame, these eggs are killed.
The researchers bred mice with an allele, or variation of a gene, called Trip13, that caused failure of egg and sperm cells during meiosis. When they silenced the Chk2 gene in these mice, the researchers reversed female infertility. By turning off this system for identifying unrepaired DNA, the researchers believe they may buy time for eggs to be repaired.
In the lab, “all the offspring from rescued oocytes are normal,” said study lead author Ewelina Bolcun-Filas, a postdoctoral researcher in the lab of John Schimenti, professor of genetics at Cornell and the paper’s senior author.
“There are genes responsible for killing defective oocytes, but we didn’t know what they were,” said Schimenti. “We wanted to identify this genetic quality-control mechanism.”
Sex cells – eggs and sperm – are produced through meiosis, a form of cell division. During meiosis, a chromosome pair from the father and another from the mother swap sections of their DNA. This recombination, which requires double-strand breaks in the chromosomal DNA, creates a new combination of male and female genes, thereby increasing genetic variation.
Errors in or lack of recombination can lead to miscarriage or such disorders as Down syndrome.
While still a fetus, most mammals produce all the eggs needed in a female’s lifetime. This pool of immature eggs is stored in the ovaries for later use. But when exposed to radiation, the pooled immature eggs are especially vulnerable to DNA damage.
During ovulation throughout reproductive years, some of those eggs undergo latter stages of meiosis. At this stage, the Chk2 protein ensures that damaged eggs do not pass faulty DNA to the next generation. By silencing the Chk2 gene, the researchers bought more time for cells to repair the DNA damage, leading to viable eggs and renewed fertility.
In the study, the researchers disrupted the Chk2 gene so the Chk2 protein was not produced. But the researchers are now working to inhibit the Chk2 protein with known drugs, which could be used in therapies to prevent infertility in women undergoing cancer treatments, Bolcun-Filas said.
The next step for this research will be to understand why the technique works for eggs but not for sperm with DNA damage. The researchers suspect that the sex chromosome differences between males (XY) and females (XX), and the different timing of meiosis in males, may play a role.
Coauthors included Vera Rinaldi and Michelle White, both Cornell graduate students in the field of biomedical sciences.
The study was funded by the National Institutes of Health and New York State Stem Cell Science program.
Media Contact
Get Cornell news delivered right to your inbox.
Subscribe