Sugar-coated vesicles prove effective in laboratory tests on deadly pathogens
By Tom Fleischman
For 30 years, conjugate vaccines have proven effective in reducing the incidence of disease caused by bacterial pathogens, including Haemophilus influenzae type b (Hib), a form of bacterial meningitis that kills thousands of infants annually in the underdeveloped world.
But manufacture of these vaccines is an expensive, highly complex, labor-intensive process that requires production of large quantities of the target pathogen, part of which gets attached – conjugated – to a protein carrier.
“This is a tried-and-true method that many pharmaceutical companies use to make vaccines for a wide range of pathogens,” said Matt DeLisa, the William L. Lewis Professor of Engineering in the Robert Frederick Smith School of Chemical and Biomolecular Engineering. “But many, including our lab, have thought that there’s got to be a better way.”
DeLisa and frequent collaborator Dave Putnam, associate professor in the Nancy E. and Peter C. Meinig School of Biomedical Engineering, have joined forces with a group at Harvard Medical School led by professor Gerald Pier to propose that better way.
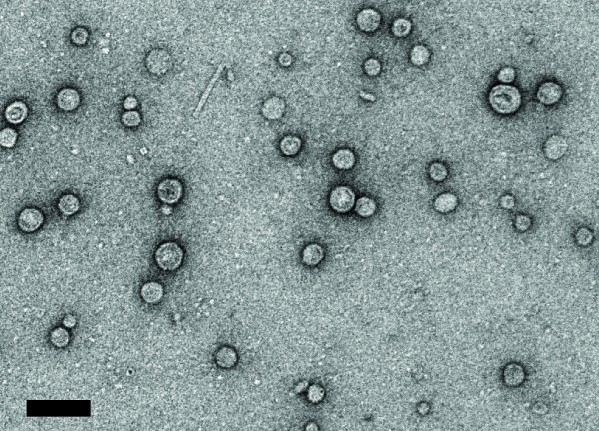
Their method uses the versatility and ease of manufacture of outer membrane vesicles (OMVs), which the DeLisa-Putnam partnership has been developing for several years as a way to deliver targeted vaccines – such as the kind Pier has been working on for years at Harvard.
Their paper, “Immunization With Outer Membrane Vesicles Displaying Conserved Surface Polysaccharide Antigen Elicits Broadly Antimicrobial Antibodies,” published March 19 in Proceedings of the National Academy of Sciences. Taylor Stevenson, Ph.D. ’17, formerly of the DeLisa Group, is lead author.
For two decades, Pier’s laboratory has focused on the role of the beta-1-6 polymer of N-acetyl glucosamine – known as PNAG – as a vaccine target. PNAG is expressed on the surface of numerous bacteria, fungi and protozoan organisms, making it an attractive target for vaccination against multiple human and animal pathogens.
“PNAG has been detected on the surface of almost every Gram-negative and Gram-positive bacterium that’s been tested – it’s everywhere,” DeLisa said. “It’s akin to a universal target on the surface of bacteria.”
Pier’s lab has been creating prototypical conjugate vaccines containing PNAG in the traditional way, but DeLisa and Putnam approached Pier and suggested their OMVs as an alternative. PNAG is bound to the outer membrane of an engineered nonpathogenic form of E. coli, and ultimately sloughs off on nanosized vesicles that can be easily harvested and used to deliver the vaccine.
Animal models infected with two distinct and potentially deadly PNAG-positive bacterial species – Staphylococcus aureus and Francisella tularensis – developed protective immunity against both when injected with the group’s OMVs.
Their findings give DeLisa hope that this method could be almost universally successful. “We predict,” he said, “that of the dozens of pathogenic bacteria that are confirmed to have PNAG on their exterior, all would be potentially targeted by the vaccines that we’ve created.”
One hurdle yet to be cleared: The laboratory strain of E. coli they use (K-12) contains an endotoxin (lipopolysaccharide, or LPS), which comes along for a ride in OMVs. The Food and Drug Administration (FDA) has limits on the amount of endotoxin a medical device or treatment can have; DeLisa said they’re working on a way to genetically engineer the bacteria to produce OMVs with dramatically less endotoxin, well below the limits set by the FDA.
“We can make the vaccines quite easily,” DeLisa said, “we can show that they’re quite effective in animal models, but a major question right now is how safe are these and can we make them safer?
In February, Cornell and Harvard jointly filed a provisional patent application for their OMV protocol.
Contributors included Tyler Moeller and Kevin Weyant, doctoral students in the field of chemical and biomolecular engineering, and Yung-Fu Chang, professor in the College of Veterinary Medicine and director of Cornell’s infectious disease research program.
This work was supported by grants from the National Science Foundation, the National Institutes of Health and the Defense Threat Reduction Agency. The researchers also made use of the Cornell Center for Materials Research, which is supported by the NSF.
Media Contact
Get Cornell news delivered right to your inbox.
Subscribe