3D growth allowed plants to move from water to land
By Krishna Ramanujan
For plant life to transition from water to land some 450 million years ago, a number of adaptions needed to occur. One innovation made by those first land plants was to grow in three dimensions, as their algal descendants only grew in two dimensions, in stringy filaments and flat mats.
For the first time, an international team of researchers has uncovered the genetic underpinnings that allowed 3D growth in land plants. The study, authored by Cornell and University of Bristol researchers, among others, was published in the Aug. 6 Current Biology.
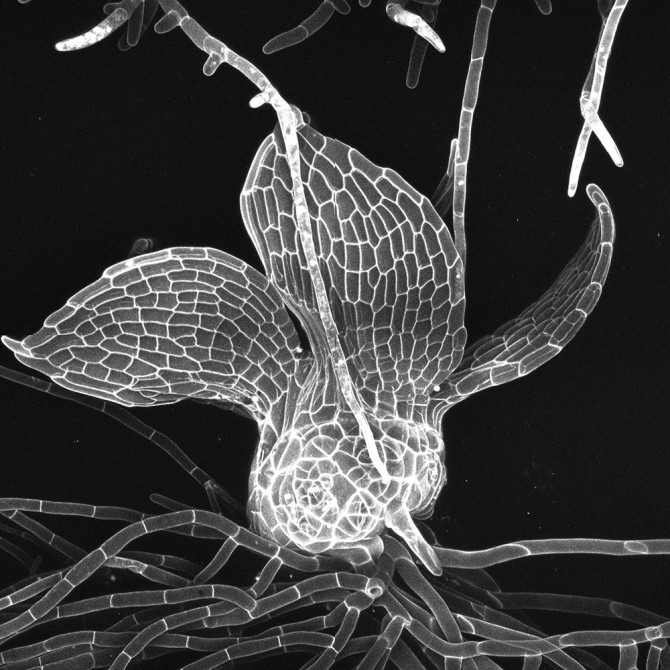
The researchers identified a number of genes that facilitate three-dimensional growth in a modern moss (Physcomitrella patens). The genes are involved in the CLAVATA signaling pathway, which includes signaling proteins known as peptides and related receptors.
“It’s clear that CLAVATA signaling is critical for carrying out the transition to 3D growth in moss, and that each of the components comprising the pathway can only be found in land-plant genomes – not in two-dimensionally growing algal lineages,” said Joseph Cammarata, a co-first author of the study and a graduate student in the lab of Adrienne Roeder, associate professor at Cornell’s Weill Institute for Cell and Molecular Biology, and in the lab of Michael Scanlon, professor in the Plant Biology Section of the School of Integrative Plant Science. Scanlon and Roeder are co-authors on the paper.
That CLAVATA signaling is found only in land plants suggests that once they colonized land, the evolution of CLAVATA signaling contributed to the formation of upright plant forms, which are better able to capture light, disperse spores and, later, seeds, Cammarata said.
When Physcomitrella moss develops from spores into an adult plant, its growth mimics the larger evolutionary pattern that enabled plant life’s move to land, where tissues oriented in 2D give rise to 3D structures. In Physcomitrella moss, a stem cell on the shoot, called a meristem, divides repeatedly, but at first in only one direction, creating flat stringy tissue, like algae. As the shoot cells continue to divide, they start growing in three dimensions.
The researchers used a variety of genetic tools and techniques to disable genes that regulate specific signaling peptides in the CLAVATA pathway. When they mutated these genes, shoot tissues formed into amorphous blobs, but only after the first few divisions, once they reached a stage of three-dimensional growth.
The CLAVATA pathway has been previously studied in flowering plants, including Arabidopsis thaliana, a model plant often used in research. That previous work focused on regulation of meristem cells, which divide to develop shoots. The current study suggests the CLAVATA pathway serves two functions in both Physcomitrella and Arabidopsis: It regulates the meristem cells as well as orientation of cells along planes (3D growth). The researchers believe regulating orientation of cell divisions may have been an ancient function of the pathway, which aided the transition to land.
“The mutants [experimental mosses] all have defects in those division planes, so we think it is critical. We think that’s the ancient function of this pathway,” Scanlon said. “We went back and looked in plants like Arabidopsis, and we saw, yeah, they also had defects in cell division planes [when the CLAVATA pathway was altered], which was previously undescribed. We think maybe the regulation of the stem cells evolved later.”
Said Cammarata: “More work in other plant lineages on CLAVATA signaling will really help us to understand more about how this pathway and land-plant architectures evolved.”
The paper’s senior author is Jill Harrison, a senior lecturer at the University of Bristol in the United Kingdom, and former research fellow at the University of Cambridge. Chris Whitewoods, co-first author, is a former graduate student in Harrison’s lab. Cammarata received his master’s from the University of Cambridge, where he also worked in Harrison’s lab.
Media Contact
Get Cornell news delivered right to your inbox.
Subscribe