New material answers call for high-frequency electronics
By David Nutt
Millions of cellphones rely on barium-strontium titanate to adjust, or “tune,” their antennae circuitry and achieve clear reception. A Cornell-led collaboration has created a new material that will bring this clarity and extra bandwidth to the next generation of cellphones and other high-frequency electronics.
The team’s paper, “Targeted Chemical Pressure Yields Tuneable Millimetre-Wave Dielectric,” published Dec. 23 in Nature Materials.
Today’s cellphones, including the newest 5G phones, operate at frequencies below 6 gigahertz (GHz), but the second wave of 5G and anticipated 6G cellular communications will require frequencies above 30 GHz, a range in which barium-strontium titanate doesn’t perform well.
“Our material gives the same performance that you get in today’s cellphone material, at 100-times-higher frequencies,” said senior author Darrell Schlom, the Herbert Fisk Johnson Professor of Industrial Chemistry. “That’s a big deal because as you increase the frequency, you get more bandwidth. So more data can flow. And people are hungry for data, especially on their cellphones.”
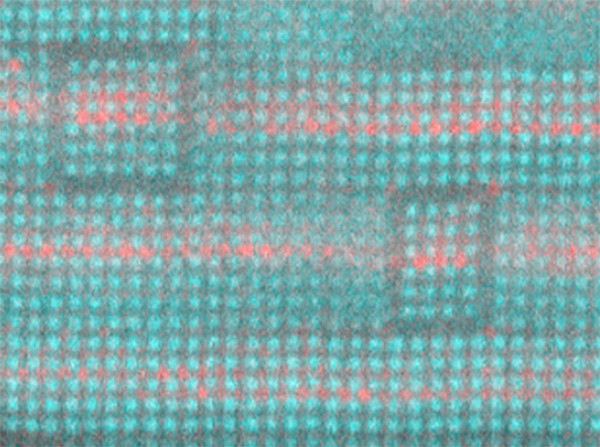
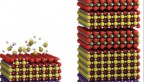
Schlom partnered with David Muller, the Samuel B. Eckert Professor of Engineering, and Craig Fennie, associate professor in applied and engineering physics. In 2013, the group used molecular beam epitaxy to create a layered strontium titanium oxide for a similar purpose. That insulator, also a tunable dielectric, boosted the performance of circuit capacitors up to about 40 GHz by removing the barium from barium-strontium titanate.
“The materials before were kind of ‘lossy,’ which means they wasted a lot of energy and turned it into heat, rather than useful tuning of the antennae. Part of the reason was it was a mixture of barium and strontium atoms that were thrown in randomly,” said Muller, a co-director of the Kavli Institute at Cornell for Nanoscale Science. “We learned that when we ordered the strontium, we got less loss. And so this time, with Craig’s guidance, instead of throwing the atoms in randomly so they just fell anywhere, we tried to keep the two different types of atoms in different layers to take away some of the randomness and remove the disorder. By removing the disorder, we’d remove the losses and the power dissipation.”
At Fennie’s suggestion, Schlom’s team reintroduced barium, in a carefully applied layer just one atom thick, to their strontium titanium oxide dielectric through a technique called targeted chemical pressure.
“I had a gut feeling that this would work and it took nearly a year of calculations to conclusively show the promise of this approach,” Fennie said. “Of course, it is one thing to put the atoms into the desired locations on the computer and simulate how such a hypothetical material will perform, and quite another to get them into those locations, which in this case happen to be positions they don’t really want to be in.”
“One of the challenges in this particular project was, we were trying to make a material that not only doesn’t exist in nature, but doesn’t want to exist in nature,” Schlom said. “And by that, I mean it’s thermodynamically unstable. So we were trying to put a layer of atoms where Mother Nature would like to find another way for those atoms to arrange. Developing the synthesis science to surmount this challenge is the magnum opus of my student Natalie Dawley’s Ph.D. research.”
The resulting material, with its tidy layer of barium, is capable of operating at frequencies up to 125 GHz, well above the desired 30 GHz for the next wave of 5G and even 6G cell service. The tunable dielectric could also be used for defense applications such as electronic spoofing, in which a signal is deployed to confuse high-frequency radar systems.
The paper’s co-lead author is Natalie Dawley Ph.D. ’19. Co-authors include doctoral student Megan Holtz, postdoctoral researcher Gerhard Olsen and undergraduate Jingshu Zhang ’14. Researchers from the National Institute of Standards and Technology, the Institute of Physics of the Czech Academy of Sciences, and the Leibniz Institute for Crystal Growth also contributed.
The research was supported primarily by the U.S. Department of Energy. Additional support was provided by the National Science Foundation and the Czech Science Foundation.
Media Contact
Get Cornell news delivered right to your inbox.
Subscribe