Study finds how stressed-out cells halt protein synthesis
By Krishna Ramanujan
Cells experience stress in multiple ways. Temperature shifts, mis-folded proteins and oxidative damage can all cause cellular stress. But whatever the form of the stress, all cells quickly stop making proteins when under pressure.
A new Cornell study unravels how cells rapidly stall protein synthesis during stress and then resume their protein-making activities once the stress has passed.
If proteins continued to synthesize during stress, cells would waste energy, and damaged proteins would build up, leading to toxicity and disease.
Previously, researchers thought that during stress protein synthesis was only controlled at the point where the translation machinery starts to read mRNA, a DNA transcript carrying protein codes.
But the new study, published online Jan. 3 in the journal Molecular Cell, reports that the protein synthesis can actually be halted midway, during the subsequent phase in the protein synthesis process, called elongation, where proteins are being made in a long chain of amino acids, like ground beef coming out of a grinder.
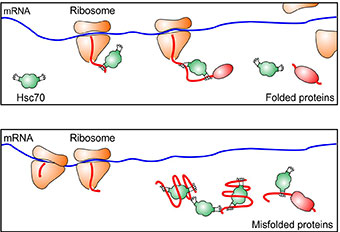
The researchers used a technique they developed to monitor the protein synthesis process that involves ribosomes, which decode mRNA and build chains of amino acids, a protein’s building blocks. They found, in the presence of stress and mis-folded proteins, the ribosomes pause during the early elongation process, when new peptides (chains of amino acids) were made but were still less than 50 amino acids long.
“We were very surprised,” said Shu-Bing Qian, assistant professor of nutritional sciences at Cornell and the paper’s senior author. “We thought it would pause everywhere, but we only found ribosomes pausing within the first 50 amino acids. We realized the translation machinery must have a mechanism for controlling trafficking in this region.”
When peptides are made, they emerge from a tunnel at the end of the ribosome that is about 30 to 50 amino acids long. “Inside the tunnel, newly formed peptides are hidden from the outer environment,” said Qian. But once they emerge, molecules called chaperones help to pull them from the ribosome.
The researchers found that under stress, chaperones were not present to pull the nascent peptides out as they emerged from the tunnel. The chaperones were instead recruited to help peptides – damaged by conditions in the cell caused by stress – refold into proteins.
“Our study shows that chaperones not only help folding but also control the ribosomes,” said Qian. “We used chemical inhibitors to inhibit the chaperones in unstressed cells, and they paused in exactly the same place.”
While Qian and colleagues looked at this process during stress caused by protein mis-folding, they believe the same process occurs no matter the source of stress.
If peptides were continuously produced during stress, they would become damaged and would accumulate, leading to toxicity and disease. Cancer cells, which grow out of control, have very high levels of chaperones for continuous protein synthesis. Researchers have developed chaperone inhibitors as a way of curbing cancer. Such inhibitors were used in this study to inhibit the chaperones in normal cells.
The study was funded by the National Institutes of Health, the Ellison Medical Foundation and the Department of Defense. Botao Liu, a graduate student, and Yan Han, a former postdoctoral associate, both in Qian’s lab, were lead authors of the study.
Media Contact
Get Cornell news delivered right to your inbox.
Subscribe