New findings explain how UV rays trigger skin cancer
By Krishna Ramanujan
Melanoma, a cancer of skin pigment cells called melanocytes, will strike an estimated 87,110 people in the U.S. in 2017, according to the Centers for Disease Control and Prevention.
A fraction of those melanomas come from pre-existing moles, but the majority of them come from sources unknown – until now.
Cornell researchers have discovered that when melanocyte stem cells accumulate a sufficient number of genetic mutations, they can become the cells where these cancers originate.
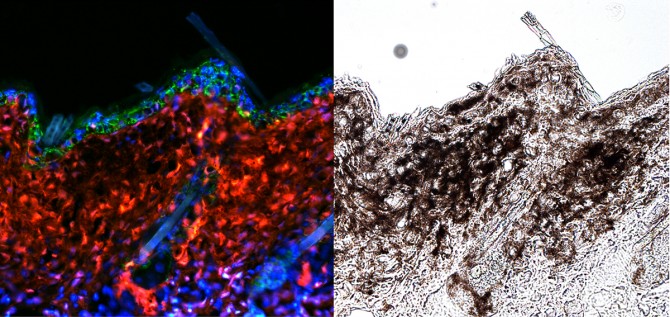
Under normal conditions, ultraviolet (UV) radiation from the sun activates melanocytes to release melanin, a pigment that protects the skin from the sun’s rays. But if melanocyte stem cells have surpassed a threshold of genetic mutations, a tumor can start to grow when those skin stem cells are activated by sun exposure.
“If you had mutations that were sufficient for melanoma, everything would be fine until you went out and got a sunburn,” said Andrew White, assistant professor of biomedical sciences at Cornell’s College of Veterinary Medicine, and senior author of a study published Oct. 12 in the journal Cell Stem Cell. Hyeongsun Moon, a postdoctoral researcher in White’s lab, is the paper’s lead author. “The stimuli that would normally just give you a tanning response could in fact start a melanoma instead,” White said.
The researchers also may have discovered a way to prevent melanomas caused by mutated stem cells. A gene called Hgma2 was suspected to become expressed in the skin under UV radiation. When expressed, Hgma2 facilitates melanocyte stem cells to move from the base of skin hair follicles to the skin’s surface (the epidermis), where the cells release melanin. Moon, White and colleagues used mice engineered with melanocyte stem cell mutations. One set of mice had the mutations, while another set with the mutations had the Hgma2 gene deleted. They then gave the mice a very low dose of UV radiation, just enough to trigger a tanning response. Mice with tumor-causing mutations and the Hgma2 gene intact developed melanomas, but the mice with mutations and the deleted gene remained healthy.
More study is needed to better understand the Hgma2 gene’s function.
“We have an actual mechanism, with Hgma2, that can be explored in the future and could be a way we can prevent melanomas from happening,” White said.
The study was supported by the Office of the Assistant Secretary of Defense for Health Affairs through the Peer Reviewed Cancer Research Program, the Cornell Center for Vertebrate Genomics, the Cornell Stem Cell Program and the National Institutes of Health.
Media Contact
Get Cornell news delivered right to your inbox.
Subscribe