Microcavities save organic semiconductors from going dark
By David Nutt
More and more electronics manufacturers are favoring organic LED displays for smartphones, TVs and computers because they are brighter and offer a greater color range.
The organic semiconductors that drive these devices are highly flexible and easily controlled. They also have the potential to be mass produced more readily than inorganic semiconductors such as silicon, which require higher temperatures for processing.
But there is a dark side to purely organic LEDs: They can be incredibly wasteful, losing up to 75% of their energy because organic semiconductors have a tendency to enter “dark states” in which they don’t emit light. These states sometimes even lead to the devices breaking down. Researchers have been looking for ways to either harness these dark states or jettison them altogether.
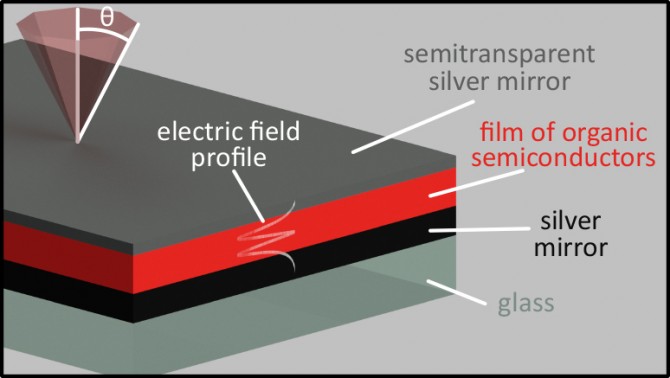
A collaboration led by Andrew Musser, assistant professor of chemistry and chemical biology in the College of Arts and Sciences, and Jenny Clark of the University of Sheffield, United Kingdom, has found a way to keep these organic semiconductors from going dark. Musserused tiny sandwich structures of mirrors, called microcavities, to trap light and force it to interact with a layer of molecules, forming a new hybrid state, known as a polariton, that mixes light and matter.This approach could lead to brighter, more efficient LEDs, sensors and solar cells.
The team’s paper, “Manipulating Molecules with Strong Coupling: Harvesting Triplet Excitons in Organic Exciton Microcavities,” published Nov. 27 in Chemical Science.
“In the LED world, people are putting huge efforts into designing these vast libraries of molecules and testing them in different device configurations to see if, by tweaking the bonds or changing energy levels, they can harvest these dark states more efficiently,” Musser said. “It’s a cumbersome, difficult battle because it’s really hard to design molecules. And you don’t necessarily know how to make them do what you want.
“So what we’ve done here is address that problem with a standard molecule, purely by putting it between these mirrors and tuning the way it interacts with light,” he said. “This suggests that, for some phenomena, we can bypass a lot of this cumbersome synthetic exploration and tune the molecules at a distance.”
Musser’s interest in polaritons began while he was studying the ways organic semiconductors can improve light-harvesting efficiency in solar cells. In that case, molecules undergo a process called singlet fission, in which they absorb one photon and split that energy into two “packets” – essentially two excited electrons – thereby doubling the photon current efficiency in the solar cell.
Musser began investigating how the reverse process can also occur, with two packets of energy combining into a single, high-energy state that can emit a high-energy photon. That led him to microcavities and the ways these simple optical structures can have a profound effect on organic material through light.
In addition to manipulating a molecule’s electronic properties for enhanced brightness, recent research has demonstrated that these structures also can be used to target specific bonds and change their chemical reactivity.
Musser said different molecules interact with light in the microcavities in different ways, and further research is needed to explore the rules that underpin their behavior.
“Right now, it serves to show that when you have these complex materials and you do something even more complicated with them – putting them between these mirrors – weird and wonderful things can happen,” Musser said.
“This work literally sheds light on dark states,” said Clark. “We’ve shown that we can use polaritons to force dark states to emit light. Apart from immediate applications for LEDs, this offers a new method for studying organic semiconductors more broadly, using previously unavailable techniques.”
Co-authors include researchers from University of Sheffield; the University of California, San Diego; the University of Cambridge; and the University of Kentucky.
The research was supported by the Engineering and Physical Sciences Research Council in the United Kingdom, the University of Sheffield, the European Commission, the European Graphene Flagship Project and the U.S. Department of Energy. The work was carried out at the Lord Porter Laser Facility in Sheffield.
Media Contact
Get Cornell news delivered right to your inbox.
Subscribe