Mapping brain repair and remodeling after stroke
By Karen Hopkin
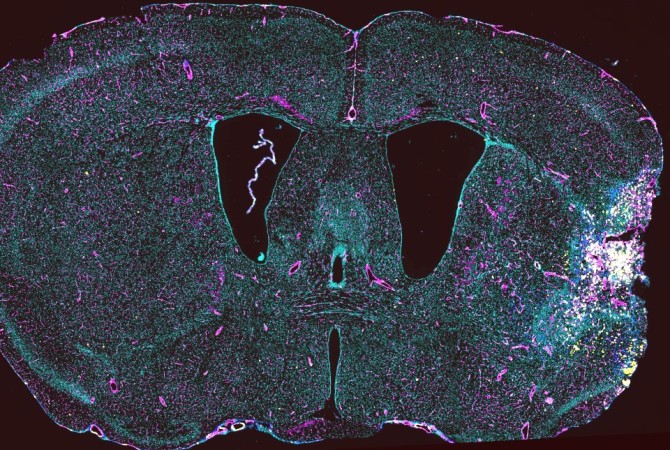
Researchers at Weill Cornell Medicine have catalogued the cellular response to stroke in a preclinical model, identifying the immune cells involved and the roles they may play in the days and weeks following a stroke.
During a stroke, loss of oxygen leads to brain damage and cell death. It also triggers a powerful inflammatory response in which the brain’s resident immune cells, along with cells recruited from the blood, infiltrate the injured tissue.
The findings, published Jan. 4 in Nature Immunology, could point toward novel approaches to fostering stroke recovery and provide insight into why therapies to control inflammation after a stroke haven’t been successful.
“Nearly every one of us knows someone who’s had a stroke. It’s a huge problem,” said senior author Dr. Josef Anrather, a professor of neuroscience and vice chair for research in the Feil Family Brain and Mind Research Institute at Weill Cornell Medicine. “But in terms of treatment, there is little a physician can do.”
Interventions that restore blood flow to the affected brain region must be administered within hours to be effective. “So most people, more than 80%, receive no therapy at all,” he said.
Understanding how immune cells contribute to repairing and remodeling the brain in the later, chronic phase after a stroke could help doctors minimize the long-term neurological consequences, including dementia and even seizures.
In 2016, Anrather and his colleagues observed that immune cells called monocytes, which are made in the bone marrow, accumulate in the brain following a stroke. Once there, they appeared to undergo a physical transformation: Some sprouted spindly arms, adopting the appearance of the brain’s resident immune cells, the microglia; others grew more amorphous and amoeba-like.
But what, if anything, did this shapeshifting have to do with their behavior?
“We became interested in knowing the function of these different structural characteristics,” said lead study author Lidia Garcia-Bonilla, the Finbar and Marianne Kenny Research Scholar in Neurology and an assistant professor of research in neuroscience at the Brain and Mind Research Institute, Weill Cornell Medicine.
They also wondered whether these cells were contributing to recovery or compounding the damage.
“There are always two sides to the coin,” Anrather said. The same cell type might be harmful in some circumstances but helpful in others. “That might be why the clinical trials of drugs that reduce immune cell infiltration into the brain and inflammation have shown no benefit for stroke.”
The most direct way to assess what a particular cell is doing is to determine which of its many genes are turned on. Working with a preclinical model, they collected immune cells at two days and 14 days after an induced stroke – the blockage of an artery in the brain. They then sequenced the RNA molecules, which encode proteins, produced by each cell. Using this approach, the researchers identified exactly each type of cell they had isolated. It also provided a readout of which genes each cell had switched on, an indication of their roles after the stroke.
The researchers first noticed that a population of microglia were rapidly proliferating. That made sense, Anrather said, “because microglia cover the territory of the brain.” When their numbers are depleted by an injury, such as stroke, the cells multiply to blanket the damaged tissue.
Then they “take out the trash,” Anrather said.
“For the brain to rebuild itself, you have to clean up, remove dead cells,” he said. Indeed, two days after the experimental stroke, the researchers detected a cadre of microglia that switch on genes involved in clearing away cellular debris.
Joining the microglia in this effort were monocytes – white blood cells that responded to the injury. “These cells circulate continuously and don’t really have a job until there is a problem, like an infection, trauma or any kind of tissue death,” Anrather said. “Then they are called in to help clean up.”
Once there, the researchers found, these monocytes transformed themselves into the type of cell that’s needed to get the job done. “They’re like little kids that get educated in the tissue,” Anrather said.
After the acute clean-up phase, the immune response was restructured toward tissue remodeling. Some cellular recruits produced growth factors triggering repair while immunological “professionals” such as T cells were called in to play a neuroprotective role.
By identifying which immune cells will heed the stroke-induced distress call, the researchers provide a novel vehicle for intervention. “Because these cells know how to get to the brain,” Anrather said, “you could use them as a shuttle and engineer them to deliver a therapeutic.”
Furthermore, understanding precisely what these cells do when they get to the brain could be key to developing treatments that can be administered weeks or months after a stroke. “Finding a way to activate the brain’s natural repair mechanism could improve the outcome for stroke patients,” Garcia-Bonilla said.
This work was supported by grants from the National Institutes of Health; the Leducq Foundation (StrokeIMPaCT Network); and the Sackler Brain and Spine Institute Research Grant.
Karen Hopkin is a freelance writer for Weill Cornell Medicine.
Media Contact
Barbara Prempeh
Get Cornell news delivered right to your inbox.
Subscribe