High-temperature superconductivity starts with nanoscale electronic oases
By Bill Steele
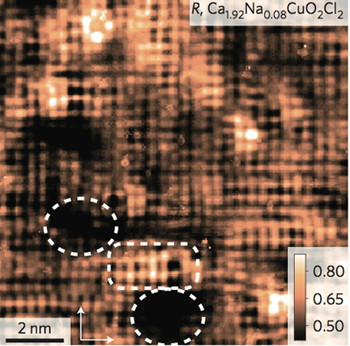
High-temperature superconductivity doesn't happen all it once. It starts in isolated nanoscale patches that gradually expand until they take over.
That discovery, from atomic-level observations at Cornell and the University of Tokyo, offers a new insight into the puzzling "pseudogap" state observed in high-temperature superconductors; it may be another step toward creating new materials that superconduct at temperatures high enough to revolutionize electrical engineering.
Using extremely precise scanning tunneling microscopes (STM) that can observe the states of electrons around atoms, an international research team led by J.C. Séamus Davis, the J.G. White Distinguished Professor in the Physical Sciences, and by Hidenori Takagi, professor of physics at the University of Tokyo, has for the first time observed how a high-temperature superconductor evolves as its chemical composition is modified. They found that as more "dopant" atoms are added, small, scattered superconducting areas, some just a few atoms across, appear. These grow until they touch and eventually fill the entire space, whereupon the entire material becomes a superconductor.
"Some theorists have imagined that this is what happens," Davis said, "but there has been no evidence until now." The research was reported May 20 in the online edition of the journal Nature Physics.
Superconductivity, in which an electric current flows with zero resistance, was first discovered in metals cooled very close to absolute zero (-273 degrees Celsius). New materials called cuprates -- copper oxides "doped" with other atoms -- superconduct as "high" as -123 Celsius.
Observations of high-temperature superconductors with the STM and other instruments show an "energy gap" where electronic states are missing. Theory says that electrons have left to join into "Cooper pairs" that can carry an electric current without interference. A puzzler for physicists is that sometimes this energy gap appears but the material still does not superconduct -- a so-called "pseudogap" phase. The pseudogap appears at higher temperatures than any superconductivity, offering the promise of someday developing materials that would superconduct at or near room temperature.
The researchers use STMs to scan a surface in steps smaller than an atom, measuring what electron energy levels are occupied and what electrons are conspicuous by their absence. They examined a series of samples of a material known as sodium-doped calcium cuprate, prepared with gradually increasing sodium content. As more sodium is added to the mix it displaces calcium atoms, changing the crystal structure and the arrangement of electrons in ways not completely understood. This particular cuprate was chosen because its simple chemistry allows fine tuning, Davis said. The phenomena observed had not been seen before because most cuprates make abrupt transitions from insulator to pseudogap to superconductor, he explained.
At a moderate level of doping, the STM finds small, scattered areas with the pseudogap signature. These areas also show a "broken symmetry" where the arrangement of electrons between copper and oxygen atoms differs between "north and south" and "east and west" in the square crystal lattice. Davis and colleagues had found this broken symmetry in earlier observations of the same superconductors.
As doping increases, these areas become larger until finally they touch, and the entire sample becomes a superconductor. It's presumed that the scattered pseudogap regions occur in the vicinity of dopant atoms, but those atoms were not observed in the current study, Davis said.
Previously, the researchers noted, it was thought that the pseudogap phase in cuprates might be in competition with superconductivity, something that had to be gotten out of the way before superconductivity could happen. This work, they said, suggests that it is beneficial -- a necessary step in the evolution of a superconductor.
The research was supported by the U.S. Department of Energy and the Japan Society for the Promotion of Science.
Media Contact
Get Cornell news delivered right to your inbox.
Subscribe