Expelled DNA that traps toxins may backfire in obese
By Carly Hodes
The body’s most powerful immune cells may have a radical way of catching their prey that could backfire on people who are overweight and others at risk for cancer, diabetes and chronic inflammation, suggests a new Cornell study.
The research describes the phenomenon and its potential cause, why it may threaten health and how to use this knowledge to develop new therapies for an array of diseases. It was published in Frontiers in Immunology this past spring.
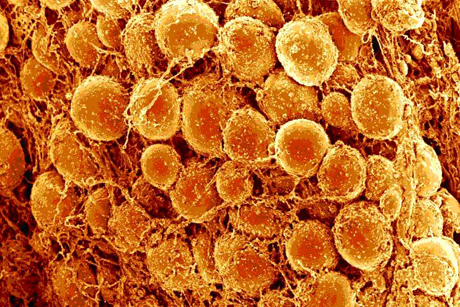
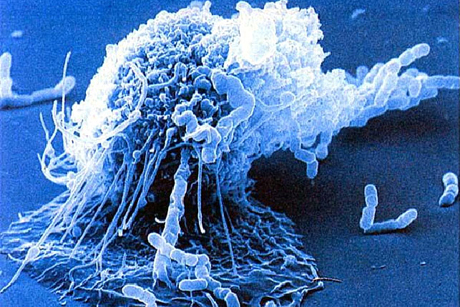
The study is the first to show that the DNA of macrophages, the biggest immune cells, can unravel and move outside the cell to snag invading pathogens. Called extracellular traps, these sticky DNA remnants can occur anywhere, but the study found a troubling number inside rafts of macrophages surrounding dead fat cells in obese mice.
In that extracellular environment, the traps feed a vicious cycle of inflammation, increasing risk of several major diseases, the scientists predict. Uncovering what causes macrophage DNA to unravel, the study included a description indicating new preventative therapies for these diseases may be near at hand.
“Our collaborator, Paul Thompson at Scripps, has developed a new drug that we have shown can block trap formation and cancer growth by inhibiting the process that triggers macrophage DNA to unravel and become traps,” said Scott Coonrod, associate professor at the Baker Institute for Animal Health at Cornell, who oversaw the study. “We envision someday using this new drug as a preventative therapy for cancer and other inflammation-related diseases.”
A chemical event called hyper-citrillunation appears to cause extracellular trap formation, according to Coonrod’s findings. It occurs when histones, which pack DNA into the nucleus, lose their electrical attraction to DNA, causing the roughly 2-meters-worth of DNA to be propelled outside the cell. While these traps normally help to clean up bacteria following infections, they also have a dark side. They are increasingly being found in diseases that do not have an infectious component, suggesting that, in some cases, traps may actually promote disease progression.
Breast cancer presents a particular concern for people who are overweight, said Coonrod’s collaborator Dr. Andrew Dannenberg at Weill Cornell Medical College. Dannenberg’s team was the first to find crown-like-structures (CLS), donut-shaped chunks of dead fat cells that are surrounded by macrophages, in human breasts. Dannenberg’s work suggests that these structures release inflammatory signals that increase the risk of breast cancer.
“One of macrophages’ jobs is to clean up dead cells,” said Coonrod. “When they come to sites with CLS to vacuum up the dead fat, the environment is full of inflammatory chemicals that promote trap formation. We looked at CLS lesions in breast tissue to see if macrophage traps were there. Our initial findings suggest that they are.”
Inflammation plays a large role in the development of cancer. The Coonrod group is currently looking into the possibility that trap production in CLS lesions promotes inflammation.
“While still in the early stages, these findings are exciting because we have a drug that can block trap production,” said Coonrod. “One could imagine that our anti-trap drug might be used one day to prevent disease by suppressing inflammation in inflammatory environments, such as breast tissue in women who are obese, thereby preventing disease progression.”
The research “Identification of Macrophage Extracellular Trap-Like Structures in Mammary Gland Adipose Tissue: A Preliminary Study,” was published in March and was supported in part by a U.S. Department of Defense Hope Scholar Award, the Breast Cancer Research Foundation and the Botwinick–Wolfensohn Foundation.
Carly Hodes ’10, MBA ’15, is a communications specialist at the College of Veterinary Medicine.
Media Contact
Get Cornell news delivered right to your inbox.
Subscribe