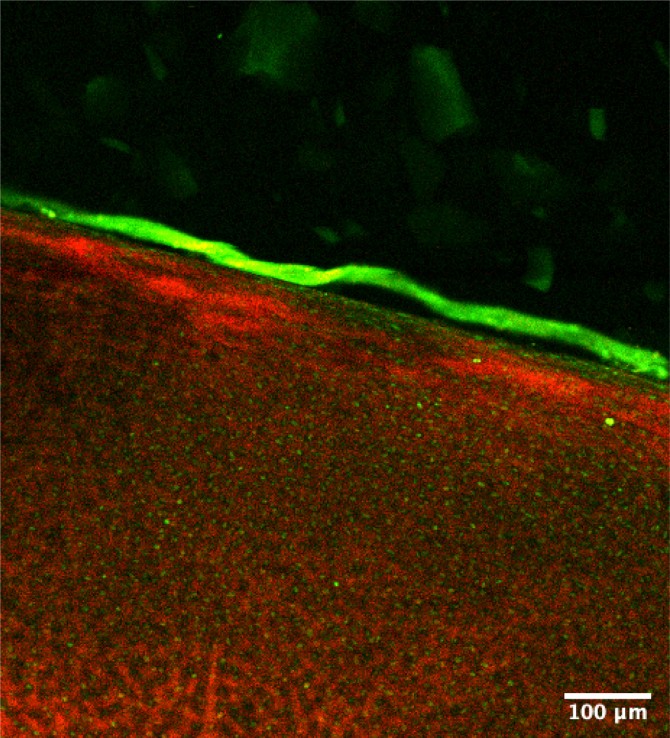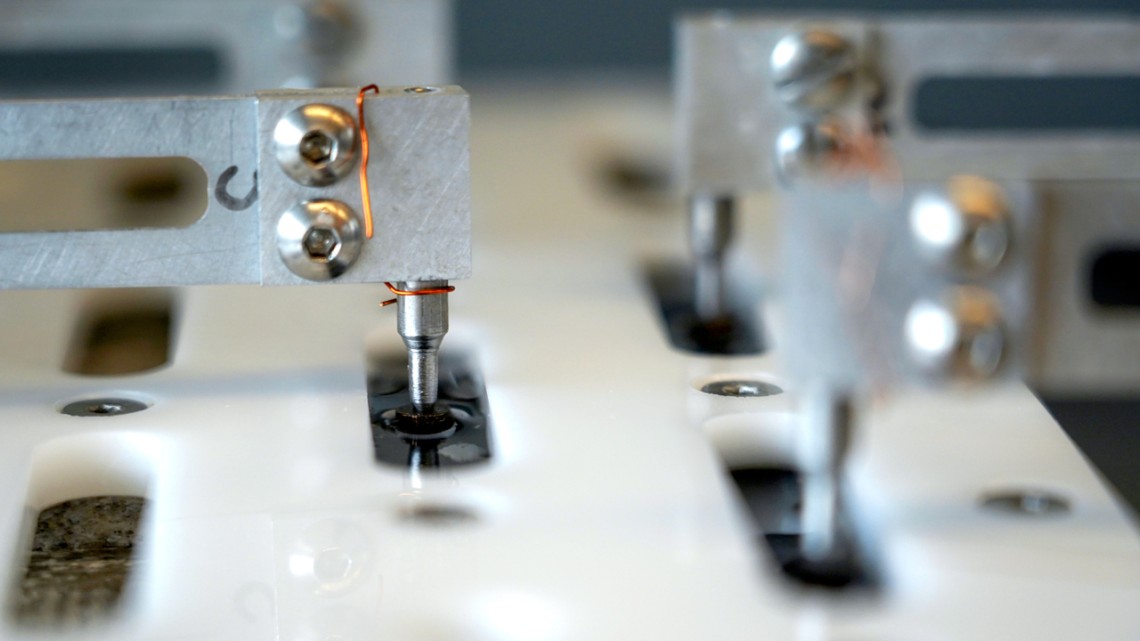
The Bonassar Research Group uses a custom tribometer to measure the frictional properties of soft materials and tissues, including biosynthetic lubricants.
Interdisciplinary group creating biolubricants to combat arthritis
By David Nutt, Cornell Chronicle
An interdisciplinary research team received a five-year, $2 million grant from the National Science Foundation to develop a new generation of biosynthetic lubricants that have the potential to treat arthritis and reduce the painful friction of artificial joints.
The Leading Engineering for America’s Prosperity, Health and Infrastructure (LEAP Hi) program, which starts Aug. 1, will be led by Lawrence Bonassar, the Daljit S. and Elaine Sarkaria Professor in Biomedical Engineering and in Mechanical and Aerospace Engineering, in collaboration with David Putnam, professor in the Meinig School of Biomedical Engineering and Smith School of Chemical and Biomolecular Engineering, Matthew Paszek, associate professor in the Smith School of Chemical and Biomolecular Engineering, all in Cornell Engineering; Heidi Reesink, Ph.D. ’16, associate professor in the Department of Clinical Sciences at the College of Veterinary Medicine; and Roberto Andresen Eguiluz, Ph.D. ’15, an assistant professor at the University of California, Merced.
The collaboration was initially spurred by an “aha” moment 10 years ago, when Bonassar attended a presentation by one of Putnam’s doctoral students describing new biomaterials to prevent surgical adhesions. One specific synthetic polymer caught Bonassar’s attention because it bore an uncanny resemblance to lubricin, a natural glycoprotein that is secreted in the synovial joint.
“Lubricin looks like a bottlebrush. It’s got a linear protein core and a whole bunch of little, short sugar side-chains on it,” Bonassar said. “Dave’s student presented a new polymer that looked a lot like that. And I thought, hey, if you’re making polymers for this other purpose, maybe we could tune the way you make those polymers to be a good lubricant for cartilage.”
For nearly two decades, Bonassar has been studying the mechanics and repair of cartilage, which, thanks to its lubricating properties, has the lowest coefficient of friction of any material ever made.
“If you think about slippery surfaces, like ice on ice or Teflon on Teflon, cartilage is a factor of 10 lower in friction than any of these things,” Bonassar said. “And the field is still figuring out why.”
Bonassar’s lab had developed specialty instrumentation that can measure these tiny friction coefficients and identify the crucial molecular components that drive cartilage’s ultra-efficient lubrication: lubricin and hyaluronic acid. Together, they form a hydrophilic complex that draws water to the tissue surface, lubricating it. In the cases of arthritis and traumatic injuries, this biopolymer layer is disturbed, which decreases the lubricating properties and increases stress on the tissue, damaging its cells.
Bonassar and Putnam successfully created a synthetic polymer that mimics the function of lubricin, and over the years their collaboration expanded to include Paszek, who specializes in glycoprotein engineering which enabled tailoring natural biopolymers to fit different applications, and Reesink, who lent her expertise in using animal models to validate the therapeutics.
“As we collaborated and began to meet regularly, it occurred to me, this is such a critical mass of people with unique skill sets,” Bonassar said. “We have the ability to work as a group in a way that is much more effective than four of us working in parallel. There’s the possibility of Dave and Matt designing new molecules, we test them for their lubricating ability in vitro, and Heidi tests them for their therapeutic potential in vivo. And we can use that to go around this design loop to continue to optimize and make better and better lubricants for different applications.”
In addition to treating arthritis and joint pain, biosynthetic lubricants could address a host of physical ailments, from dry eyes and skin abrasions to inflammatory bowel disease and certain digestive and reproductive issues – essentially anything involving mucosal layers that are unable to localize water to a surface.
“We hope that five years from now we’ve got great candidates for injectable treatments for arthritis, that we have compelling data in animals to say that these could move forward in the developmental process, and that we have general paradigms we can use and export to think about tailoring different lubricants for other kinds of medical applications,” Bonassar said.
Media Contact
Get Cornell news delivered right to your inbox.
Subscribe