Structure of protein that helps feed cancerous tumors is deciphered at Cornell University, possibly leading to more effective drugs
By Blaine Friedlander
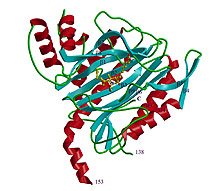
For some years now, cancer researchers have known that cancerous tumors are fed by nutrients and oxygen through blood vessels generated by endothelial cells. Now the hope is to develop drugs to prevent the cells from forming the blood vessels, thus starving the tumors.
Researchers working at Cornell University have brought this goal closer by deciphering the three-dimensional structure of a protein that helps cells build new blood vessels. The researchers also have discovered how a drug now in clinical trials binds to the protein methionine aminopetidase-2, or MetAP-2, for short.
Writing today (Nov. 13, 1998) in the journal Science, a research team led by Jon Clardy, Cornell's Horace White Professor of Chemistry and Chemical Biology, and Shenping Liu, Cornell visiting scientist, note that this new understanding of MetAP-2 might help chemists design more effective drugs.
"Our structure provides a better basis for designing drugs against cancer," says Liu. Because the protein, MetAP-2, was specifically shown to bind to the drug, he says, the research could pave the way for anti-cancer drugs with fewer side effects. The more specific the drug, the fewer the side effects, he says.
The researchers focused on the effects of fumagillin (pronounced foo-MAA-jill-in), a small organic molecule discovered from the fungus Aspergillus fumigatus. Earlier research had first discovered that fumagillin inhibits the formation of new blood vessels, a process called angiogenesis, and that it inhibits MetAP-2.
A semi-synthetic drug, TNP-470, a fumagillin derivative, currently is undergoing human clinical testing as an anti-cancer agent. By uncovering the three-dimensional structure of the targeted protein, MetAP-2, the new results indicate precisely how the drug binds to the protein.
Because TNP-470 is broken down quickly in the body, researchers are trying to develop improved versions. The Cornell research, by showing precisely how the drug fits into the protein, is an important step for pharmaceutical designers to synthesize better drugs, Clardy says.
Interfering with the blood flow into the tumor prevents growth. However, Clardy warns, "We need to be cautious and not get our hopes too high." Several research groups are trying to figure out how MetAP-2 inhibits the formation of new blood vessels, he says, and the new drugs will take years of development and testing.
Clardy and his colleagues determined the three-dimensional structure of human MetAP-2 using X-ray crystallography at MacCHESS, the world-renowned Macromolecular Diffraction Facility at Cornell High Energy Synchrotron Source (CHESS). The synchrotron produces high-intensity, high-energy X-ray beams for scientific research.
MacCHESS is supported by the National Institutes of Health for research in drug design, protein and virus crystallography and data collection from synchrotron sources. CHESS is supported by the National Science Foundation.
The research, "Structure of Human Methionine Aminopeptidase-2 Complexed with Fumagillin," appears in the Nov. 13 journal Science. It was authored by Clardy; Liu; Joanne Widom, Cornell chemistry researcher; Craig Crews, Yale University professor; and C.W. Kemp of Kemp Biotechnologies, Frederick, Md. Funding for the research was provided by U.S. Public Health Service grants.
Media Contact
Get Cornell news delivered right to your inbox.
Subscribe