Fantastic voyage: Tiny pharmacies propelled through the body could result from Cornell breakthrough in molecular motors
By Roger Segelken
Coupling the organic and inorganic, biological engineers at Cornell University have demonstrated the feasibility of extremely small, self-propelled bionic machines that do their builders' bidding in plant and animal cells, including those in humans.
Such machines could travel through the body, functioning as mobile pharmacies, for example, dispensing precise doses of chemotherapy drugs exclusively to cancer cells.
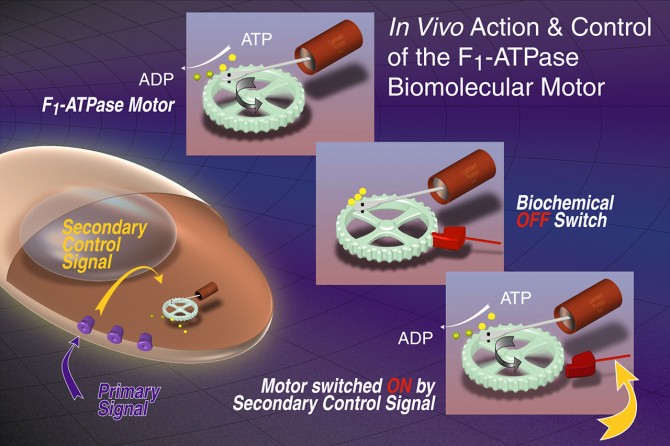
The engineers' breakthrough is in integrating a living molecular motor with a fabricated device at the "nano" scale, a few billionths of a meter in size. The first integrated molecular motor, a molecule of the enzyme ATPase coupled to a metallic substrate with a genetically engineered "handle," ran for 40 minutes at 3 to 4 revolutions per second, Carlo Montemagno and George Bachand report in the September issue of the journal Nanotechnology (Vol. 10, No.3).
The ATPase molecules were produced by Escherichia coli bacteria that were genetically engineered to include a gene sequence from the thermophilic bacterium Bacillus PS3. With further genetic manipulation, the Cornell engineers expect E. coli to turn out ATPase molecules with tiny propellers -- making each a kind of nano-motorboat — as well as other useful structures, predicts Montemagno, an assistant professor of agricultural and biological engineering.
The researchers concede they have not yet created an intelligent nanomechanical machine, saying, "We have succeeded in establishing biological and nanofabrication platforms for the production of organic/inorganic hybrid nanoelectromechanical systems (NEMS), but we have a long way to go before it's safe to turn these little machines loose in the human body." For example, Montemagno says, engineers need to determine the impact of waste products, such as heat and protons, on the motors' performance and their surrounding environment.
"We believe this is a significant step toward the seamless integration of nanoscale technologies into living systems and to the creation of organic/inorganic intelligent systems," Montemagno said. He also envisions ATPase motors pumping fluids, opening and closing valves in microfluidic devices and providing mechanical drives for a new class of nanomechanical devices. The Cornell work with ATPase motors recently was cited by Discover magazine as one of the most promising new technologies of 1999.
Molecular motors are hardly new. For billions of years they have been nature's way of accomplishing life's essential tasks at the atomic level. The ATPase molecular motors are found in the membranes of mitochondria, the microscopic bodies in the cells of nearly all living organisms, as well as in chloroplasts of plant cells, where the enzyme is responsible for converting food to usable energy. The moving part of an ATPase is a central protein shaft (or rotor, in electric-motor terms) that rotates in response to electrochemical reactions with each of the molecule's three proton channels (comparable to the electromagnets in the stator coil of an electric motor).
ATP (adenosine triphosphate) is the fuel for the molecular motor's motion. Energy becomes available when atomic bonds between phosphate atoms are broken during hydrolysis to convert ATP into ADP (adenosine diphosphate). During ATP hydrolysis, the tail rotates in a counterclockwise direction; it rotates clockwise during ATP synthesis from ADP.
The Cornell engineers tagged the ATPase molecule's rotor with fluorescent microspheres that are 1 micron (1 millionth of a meter) in diameter and observed microsphere movement with a differential interferometer and with a CCD (charge-coupled device) kinetics camera.
The "handle" for attaching the ATPase motor to the nanofabricated metallic substrates is a synthetic peptide composed of histidine and other amino acids. The histidine peptide allows the molecular motors to adhere to nanofabricated patterns of gold, copper or nickel -- the three standard contact materials in integrated circuits. The patterned metal substrates were created by evaporative deposition at the Cornell Nanofabrication Facility, which specializes in creating structures a few nanometers in size.
Besides demonstrating that an organic molecular motor and an inorganic, nanofabricated device can be integrated, the Cornell engineers note, their system provides a platform for studying fundamentals of ATPase's operation, which is not fully understood. Their device will have more brawn than brains until the molecular motors are attached to more advanced nanofabricated devices that can provide directions.
"Our long-term objective is to utilize the best attributes of the organic and inorganic worlds for NEMS that are powered by biological motors and chemical energy sources," Montemagno said. "For a technology that wasn't expected to produce a useful device before the year 2050, I think we've made a pretty good start."
Studies of nanomechanical devices powered by biomolecular motors are supported at Cornell by grants from the National Science Foundation and the Department of Energy. Montemagno is a faculty member of Cornell's College of Agriculture and Life Sciences and Bachand is a research associate in his engineering laboratory.
Media Contact
Get Cornell news delivered right to your inbox.
Subscribe