New device isolates most aggressive cancer cells
By Anne Ju
Not all cancer cells are created equal – some stay put in the primary tumor, while others move and invade elsewhere. A major goal for cancer research is predicting which cells will metastasize, and why.
A Cornell cancer research team is taking a new approach to screening for these dangerous cells, using a microfluidic device they invented that isolates only the most aggressive, metastatic cells.
“The approach we’ve taken is a reverse approach from what is conventionally done,” said Cynthia Reinhart-King, associate professor of biomedical engineering and senior author of the recently published Technology Journal paper describing the research. “Instead of looking at what molecules are being expressed by the tumor, we’re looking for the phenotype – that is, the behavior – of individual cells first. Then we can determine what molecules are causing that behavior.”
Typically, searching for biomarkers of metastasis has focused on screening for certain molecules or genes expressed by large numbers of migrating cancer cells. The problem is that it’s easy to miss subtle differences in the tiny subpopulations of cells that are the most aggressive.
Taking, for example, 100 tumors and seeking out molecular biomarkers for metastasis, one particular molecule might be identified as being “upregulated” in those tumors, Reinhart-King said. But it’s not the whole tumor expressing that particular molecule – some cells express the biomarker and some do not.
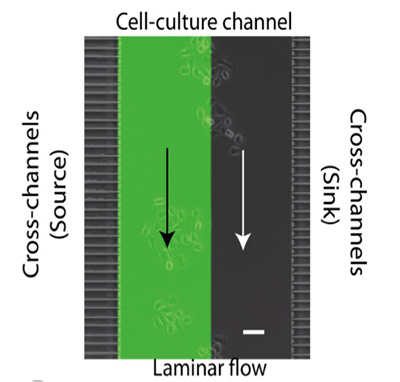
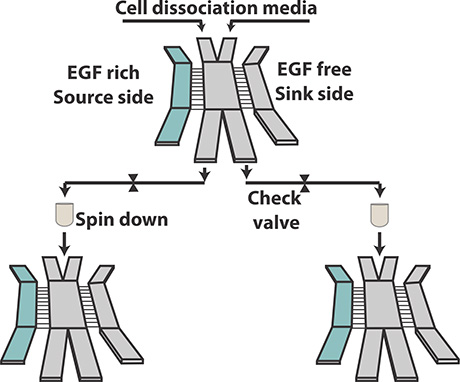
The researchers decided to first sort cells with the most aggressive behavior, and analyze only them for molecular changes. Their innovation is a microfluidic device that contains side channels to wash out the less aggressive cells, while herding the more aggressive ones into a separate channel.
For their proof-of-concept, the researchers screened for cells with migratory responses to Epidermal Growth Factor, for which the receptor is known to be present in most human cancers and is tightly linked to poor prognosis.
“The thing we’re most excited about, in addition to the physical device, is the conceptual framework we’re using by trying to shift gears and screen for cells that are causing the worst parts of the disease,” Reinhart-King said. The device could also be used in other applications of tissue engineering, inflammation and wound healing.
King collaborated with co-author Michael King; the husband-and-wife team are project leaders in the Cornell Center on the Microenvironment and Metastasis, a National Cancer Institute Physical Sciences-Oncology Center, which supported the work. Authorship includes former postdoctoral associate Saumendra Bajpai and graduate student Michael Mitchell.
Media Contact
Get Cornell news delivered right to your inbox.
Subscribe