New imaging method developed for lipid with many functions
By Krishna Ramanujan
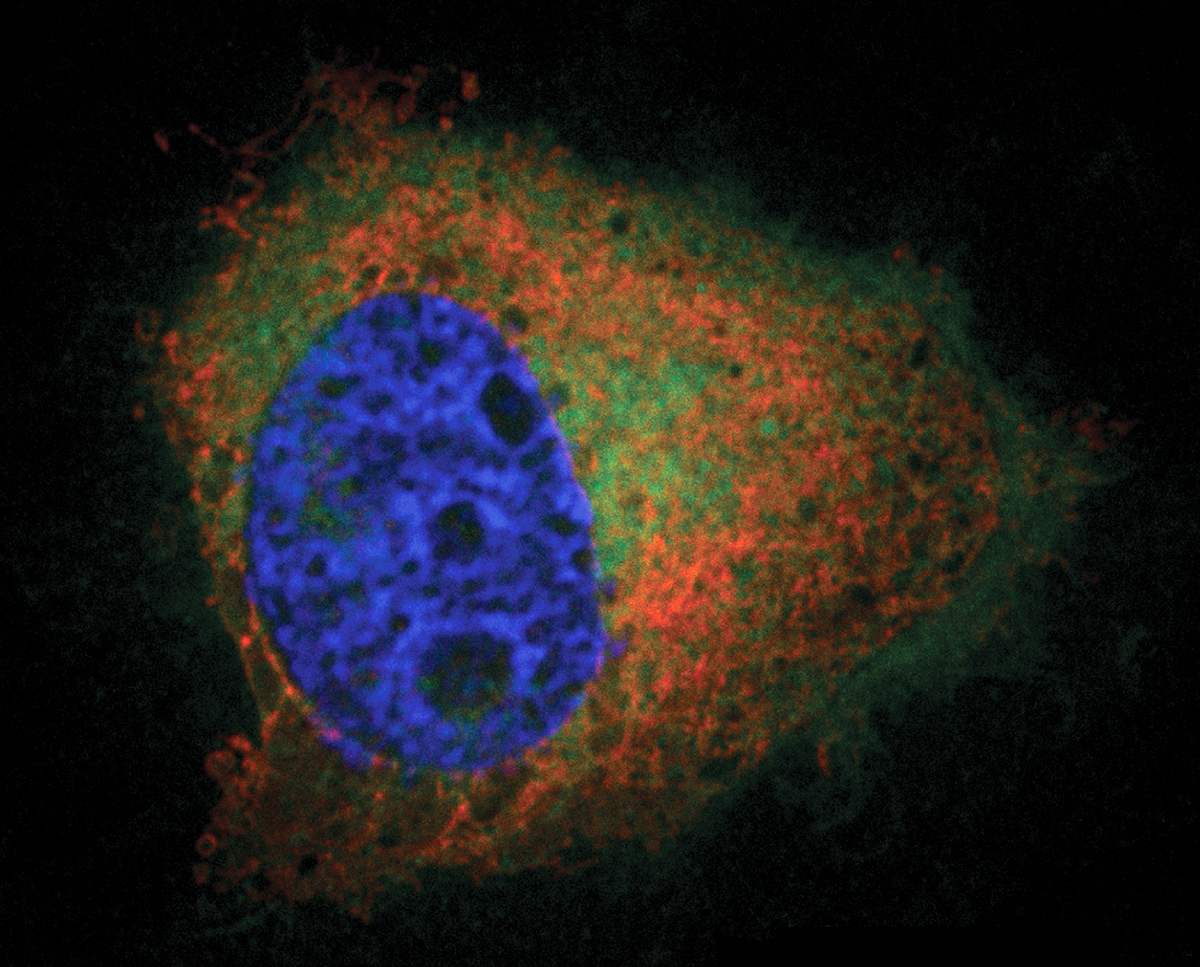
An essential molecule in cells, called phosphatidic acid (PA), is at the center of a cellular biology mystery.
This lipid, or fatty molecule, is a jack-of-all-trades – based on context, it can cause cells to move, divide or commit suicide. Elevated levels of PA have also been observed in many types of cancer as well as autoimmune and neurodegenerative diseases.
But the lipid has been hard to study, because there have been no good tools for imaging where it gets produced – until now.
Cornell researchers report in a study published online Sept. 16 in the journal Angewandte Chemie a new method for imaging PA, which could solve the mystery of how it plays so many roles.
“Our imaging approach enables us to directly visualize in cells the locations where the lipid is produced,” said Jeremy Baskin, assistant professor in the Department of Chemistry and Chemical Biology and the Weill Institute for Cell and Molecular Biology and the paper’s senior author. Timothy Bumpus, the paper’s first author, is a graduate student in Baskin’s lab.
“Based on what is currently known about the biology of PA, we believe that where PA is produced has tremendous impact on subsequent downstream biology that occurs,” Baskin said.
Researchers can now take this new imaging technique and apply it in different cell lines (cultured cells used for study) and in different biological contexts. For example, researchers could discover where PA is produced and the subsequent cellular behavior when immune cells are stimulated with antigens, or when neurons are stimulated by neurotransmitters, or when cancer cells divide or metastasize, Baskin said.
The researchers used 21st-century chemistry to add a fluorescent tag to the lipid to image it. When cells need to quickly make large amounts of PA, an enzyme called phospholipase D (PLD) facilitates that process. Under normal circumstances, PLD uses water molecules to break a bond in another lipid to make PA.
Years ago, scientists realized that alcohols – such as ethanol, found in alcoholic drinks – could be used instead of water to make an analog of PA. Bumpus and Baskin took advantage of that knowledge and a process developed in 2002 called a “click chemistry reaction,” in which two chemical groups react with another to create an irreversible bond while excluding potential competing reactions.
“The two components that are meant to react with one another will do so in a sea of other competing reactive groups, such as in an extract of a whole cell or in the context of an intact cell,” Baskin said.
In a two-step process, the researchers used an ethanol-like molecule that attaches to PLD, and they used a click reaction to attach a fluorescent tag to PA when it is produced. The tag allows them to image PA at the moment and location where it is created, using fluorescence microscopy.
In future work, the researchers will use the imaging technique to explore the basic biology of PA and study its roles in cancer and neurodegenerative diseases. Also, from an engineering perspective, the click reaction with PLD may allow bioengineers to attach other components onto PA to remodel or re-engineer membranes inside of cells and change their properties and functions.
The study was supported by the National Institutes of Health.
Media Contact
Get Cornell news delivered right to your inbox.
Subscribe