Cryogenic microscopy reveals atomic shifts of a manganite
By Tom Fleischman
Recent work from the lab of Lena Kourkoutis, assistant professor of applied and engineering physics, describes a new approach to characterizing and understanding exotic charge-ordered phases in a manganite.
Charge order is a modulation of a material’s electron density and is associated with unconventional phenomena, such as superconductivity. But those types of phenomena typically occur at ultra-cold temperatures, and the lab’s previous work looked at the material in its ordered phase at room temperature.
Kourkoutis’ latest work sheds light on the material’s nanoscale structure changes where the “fun” happens – at super-cold temperatures.
Graduate student Ismail El Baggari is lead author of “Nature and Evolution of Incommensurate Charge Order in Manganites Visualized with Cryogenic Scanning Transmission Electron Microscopy,” published Jan. 30 in Proceedings of the National Academy of Sciences.
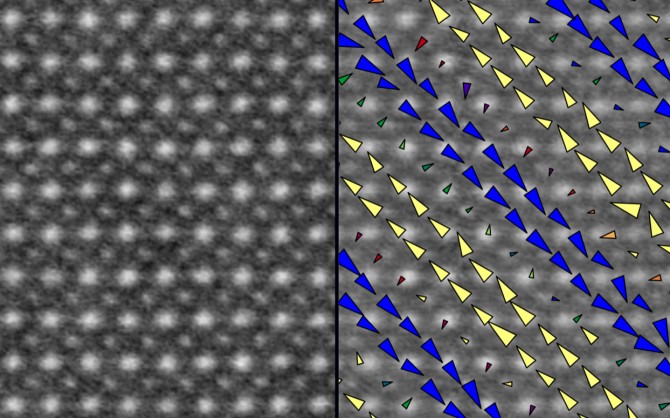
Kourkoutis noted in the group’s earlier work that, through the use of scanning transmission electron microscopy (STEM), they observed atomic scale period lattice displacements (PLDs) – ever-so-slight shifts in the material’s atomic makeup, which can lead to fundamental shifts in a material’s properties.
This work takes that ability one step further by cooling the material – in this case, a manganite comprising bismuth, strontium, calcium, manganese and oxygen – to near the temperature of liquid nitrogen (93K, or about minus-180 Fahrenheit).
They chose that specific manganite because charge ordering occurs in it at room temperature, so they can track the relevant changes in the material when supercooled. And the state-of-the-art microscope housed in the Cornell Center for Materials Research allowed for cryogenic STEM.
“In the past, you were limited to room temperature,” El Baggari said. “You look at the sample at room temperature, and then you did some other measurements, and you try to correlate the properties. Now we can provide an atomic-scale picture, at both room temperature and at those temperatures where interesting things happen.”
Using cryogenic STEM, the group observed a periodic arrangement of the atomic shifts (or stripes) in the manganite and compared them to measurements taken at room temperature. The group found that, at both temperatures, the stripes had the same periodicity at very small scales.
But by imaging larger areas (tens of nanometers) of the material, the researchers found disorder and breaking of stripes that strongly depended on the temperature. These spots of disorder cause a change in the repetition pattern of the stripes and affect the global properties of the material across temperatures.
These results, the researchers say, pave the way to understand the underlying structure of charge-ordered states and other complex phenomena, including superconductivity and metal-to-insulator transitions.
“We can now observe how the atomic lattice changes when you go from, for example, a metal state to an insulator state,” Kourkoutis said. “And we can track it with picometer [one-trillionth of a meter] precision.”
Future work will involve tracking the material at many points between liquid nitrogen and room temperatures. “We will be able to see the phase transition happen,” El Baggari said.
“In the past, we could see it through averages, doing measurements and inferring a lot of interesting phenomena that occur,” he said. “But being able to see it is another thing – and seeing is believing, right?”
Also contributing to this work was Benjamin Savitzky, a graduate student in Kourkoutis’ lab, Robert Hovden, former postdoctoral researcher and now an assistant professor at the University of Michigan, and Sang-Wook Cheong’s group at Rutgers University.
This work was supported by grants from the Air Force Office of Scientific Research, the Packard Foundation, the Moore Foundation and the National Science Foundation. The researchers made use of the Cornell Center for Materials Research, which is supported by the NSF’s Materials Research Science and Engineering Centers program.
Media Contact
Get Cornell news delivered right to your inbox.
Subscribe