Research explains nature of key plant-fungus interfaces
By Aaron J. Bouchie
For millions of years, plants and fungi have exchanged crucial nutrients such as phosphate and fatty acids. These symbiotic relationships are extremely important to the growth and survival of both organisms, but the mechanism by which this exchange happens has been poorly understood.
Now, researchers at the Boyce Thompson Institute (BTI), located on the Cornell campus, have uncovered structural networks of tubules at the plant-fungal interface that could shed light on the mechanisms of this natural partnership. A better understanding of this process could eventually help reduce the use of fertilizer in agriculture.
Findings were detailed in “Extensive Membrane Systems at the Host–Arbuscular Mycorrhizal Fungus Interface,” published Feb. 8 in Nature Plants.
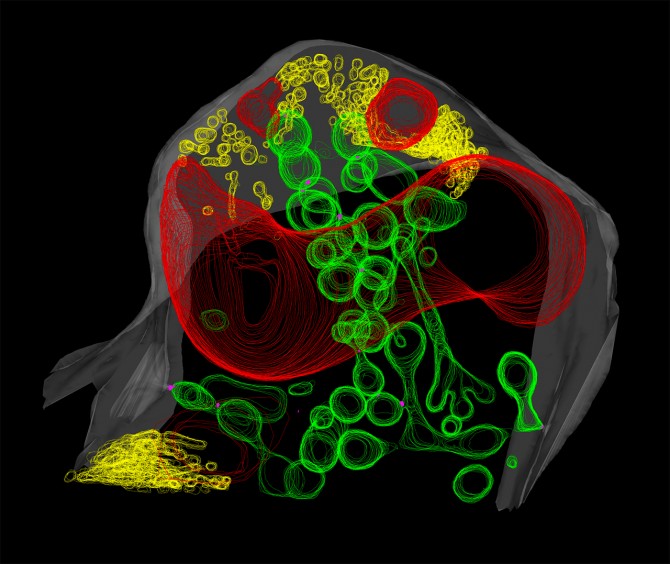
An estimated 80 percent of vascular plant families form symbioses with a type of soil fungi called arbuscular mycorrhizal fungi. The fungi penetrate the outermost cells of a plant’s roots and grow intricate branchlike structures called arbuscules. Each host plant cell then grows a membrane that envelops an arbuscule, and nutrient exchange takes place within the space between the plant membrane and the fungal cell wall.
In an attempt to understand this exchange, BTI faculty member Maria Harrison and postdoctoral scientist Sergey Ivanov used advanced electron microscopy techniques to image arbuscules present in the roots of the legume Medicago truncatula colonized by the fungus Rhizophagus irregularis.
The researchers were surprised by the results.
“[Previous studies] indicated that the material around the fungus but inside the plant membrane would be an amorphous matrix of carbohydrate material,” said Harrison, the paper’s corresponding author and an adjunct professor in Cornell’s College of Agriculture and Life Sciences.
Instead, the researchers found a network of round, tubular and dumbbell-shaped structures made of lipid membranes, nearly all of which appeared to connect back to the plant cell’s membrane.
The researchers were further surprised to find another network of membrane tubules in the space between the fungal cell membrane and the fungal cell wall.
“It was totally unexpected to see such an extensive proliferation of fungal membrane, particularly knowing that the fungus is starving for lipids,” said Ivanov, the paper’s lead author.
Harrison and Ivanov speculate that the networks are related to the transfer of lipids.
“Somehow lipids are released from the plant cell and fed to the fungus, and we wondered how they move through what we thought was an aqueous matrix between the plant cell membrane and the fungal cell wall,” Harrison said. “But maybe this space isn’t so aqueous after all, and perhaps this membrane-rich environment facilitates the movement of lipids between the organisms.”
Given the plant and fungal membrane networks’ close physical proximity to each other, the fungal network could be involved in lipid absorption in order to optimize the process. The researchers suspect the network is not involved with transferring phosphate to the plant because the membrane networks are more abundant near the larger branches of the arbuscule, whereas phosphate uptake likely occurs near the smaller branches.
Harrison believes that newer technologies are to thank for finding these networks of tubules. “High-pressure freezing fixation of samples gives better membrane preservation than older techniques. I think that is the reason that these extensive membranes were not seen before,” she said. “Plus, 3D electron tomography is very powerful and let us visualize the networks, which didn’t look connected on 2D images.”
Also contributing was R. Howard Berg, director of the Integrated Microscopy Facility at the Donald Danforth Center in St. Louis. Ivanov worked closely with Berg, who has expertise with cryofixation and electron microscopy, and Jotham Austin II at the University of Chicago, who is an expert in tomography.
Financial support for this project was provided by the National Science Foundation and by the TRIAD Foundation.
Aaron J. Bouchie is a science writer at the Boyce Thompson Institute.
Media Contact
Get Cornell news delivered right to your inbox.
Subscribe