Cell-free biotech could drive COVID-19 therapeutics
By David Nutt
A biomanufacturing company spun out of Cornell research is seeking to rapidly translate an antibody therapy against COVID-19 by using cell-free biotechnology based on glycoengineered bacteria. And it could scale up the production 10 times faster than conventional methods.
The company, SwiftScale Biologics, was co-founded by Matt DeLisa, the William L. Lewis Professor of Engineering in the Robert F. Smith School of Chemical and Biomolecular Engineering, and his longtime collaborator, Michael Jewett, a professor of chemical and biological engineering at Northwestern University.
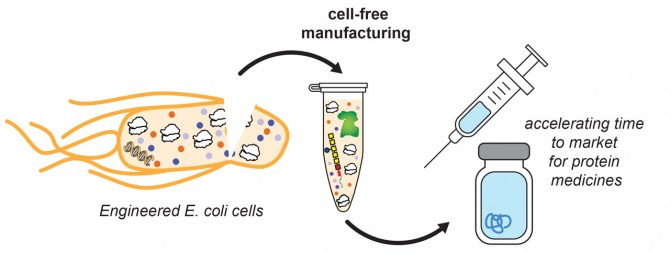
DeLisa’s research group focuses on engineering biological machinery in cells, and in 2018 they pioneered a method for cell-free manufacturing of glycosylated proteins – proteins with a carbohydrate attachment. The combination of DeLisa’s protein glycosylation technologies and Jewett’s knack for developing cell-free protein expression systems has the potential to create glycosylated protein drugs such as therapeutic antibodies, and led to the launch of their company last year.
“SwiftScale is pioneering an innovative antibody development and manufacturing platform that can significantly accelerate the normal timelines for translation of antibody treatments,” said DeLisa, who also directs the Cornell Institute of Biotechnology. “All the technologies are based off of laboratory strains of E. coli, a very simple, very fast, very customizable chassis.”
The low-cost, rapid manufacture of therapeutic antibodies could be pivotal in combating the spread of COVID-19. The conventional means for manufacturing antibody drugs relies upon the use of mammalian cell lines, specifically Chinese hamster ovary (CHO) cells. Production can take nine months or more. SwiftScale’s use of cell-free lysate derived from E. coli bacteria could shrink that timeline down to a month, which amid a pandemic could be “game changing,” DeLisa said.
“Bacteria cells do everything faster than CHO cells. They grow faster, they divide faster, they produce proteins faster,” DeLisa said. “The historical limitation has been that E. coli cells do not naturally produce any glycoproteins. What we’ve done in my lab is to equip E. coli with the machinery for installing complex carbohydrates onto proteins, which now opens up the opportunity to use these bacterial cells or their lysates for making glycoprotein products such as monoclonal antibodies.”
SwiftScale intends to produce antibody therapies that target cancer, but as COVID-19 emerged over the last several months, the company realized it could leverage its rapid, adaptable technology to potentially treat those infected with the virus instead. They have partnered with a biotherapeutics company, Centivax, that has identified several lead antibody candidates it believes could be used against the virus.
Centivax is planning to begin a phase I/II clinical trial in late July, with SwiftScale ramping up its capabilities to produce 100,000 doses a month for 10 months if the trial is successful.
“There are a lot of companies rising to the challenge and trying to provide solutions in the drug and vaccine space,” DeLisa said. “It’s been said we need as many ‘shots on goal’ as we can get. My hope is, collectively, all of these companies, SwiftScale included, will be providing enough of those shots on goal that somebody will score.
“And maybe the lessons learned from this particular catastrophic event could be applied in the future,” he said, “so that we don’t end up in a situation quite as devastating as the one that we’re dealing with right now.”
Media Contact
Get Cornell news delivered right to your inbox.
Subscribe