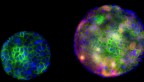
Stem cells shown to delay their own death to aid healing
By Melanie Greaver Cordova
Already known for their shape-shifting abilities, stem cells can now add “death-defying” to their list of remarkable qualities.
A new study shows how stem cells – which can contribute to creating many parts of the body, not just one organ or body part – are able to postpone their own death in order to respond to an injury that needs their attention. The study was done in planarians, which are tiny worms used as model organisms to study regeneration because of their ability to recover from any injury using stem cells.
“Planarian stem cells, even when challenged and under a lot of duress, will still respond to an injury by delaying death,” said Divya Shiroor, first author and a graduate student in Dr. Carolyn Adler’s lab, in the College of Veterinary Medicine.
The study, published May 7 in Current Biology, is the first to demonstrate this reaction in planarians.
The research team exposed planarians to radiation, then subjected half of them to injury. Radiated worms that had not been injured experienced predicted levels of stem cell death. Stem cells of the injured worms, however, survived, gathering around the site of the wound and postponing their deaths to mount a response.
“We show that this inevitable radiation-induced cell death can be significantly delayed if animals are injured soon after radiation exposure,” said Shiroor.
This could have important implications for cancer research and therapies, particularly when examining chemotherapy and surgery options for patients.
“By understanding how injury prompts planarian stem cells to withstand radiation,” Shiroor said, “we hope to identify genes that, if shared with mammals, could perhaps help hone existing therapies.”
Planarians are commonly used in basic research because of their similarities to humans. Like humans, planarians have stem cells, similar organs and similar genes, but are much more adept at responding to injury, thanks to their higher volume of stem cells and lack of a developed immune system, which in humans complicates the healing process.
“This really simplifies the process of understanding the effects of both injury and radiation on stem cells, and allows us to study it directly without being hampered by parallel processes integral to wound healing, such as inflammation, that get simultaneously triggered in mammals,” Shiroor said.
By uncovering the mechanisms that govern stem cells after wounding in a system like planarians, researchers could also apply this knowledge when engineering stem cells to respond similarly in the human body.
Labs have many ways to understand how planarians use stem cells to successfully recover and regenerate, but the Adler lab’s combination of radiation and injury to identify a novel stem cell response is unique. The researchers plan on digging deeper to understand how the stressed stem cells know that there is an injury and what role other cells may play in their response.
“We have identified a key gene that is required for stem cell persistence after radiation and injury,” Shiroor said, “and we plan on using this as a stepping stone for further exploration.”
Melanie Greaver Cordova is managing editor at the College of Veterinary Medicine.
Media Contact
Get Cornell news delivered right to your inbox.
Subscribe