Professor Susan Daniel, right, works with students Jun Yan ’16, left, and Lakshmi Nathan, M.S. ’17, Ph.D. ’19, in the laboratory.
Susan Daniel wields biomolecular weapons to fight COVID-19
By Chris Woolston
Years before the novel coronavirus turned into a global pandemic, Susan Daniel was already looking for ways to defeat it.
Daniel, professor in the Robert Frederick Smith School of Chemical and Biomolecular Engineering in the College of Engineering, had been creating models of the cell membrane to gain new insights into the virus SARS-CoV-1. It is a precursor and close relative of the new coronavirus, SARS-CoV-2, which causes COVID-19.
A dangerous virus in its own right, SARS-CoV-1 infected more than 8,000 people around the world in 2002 and 2003. That particular outbreak has long faded, but Daniel was intrigued by this virus, known as SARS 1, and wanted to know exactly how it enters its host’s cells, a crucial step toward infection. The work was scientifically interesting – these viruses have a lot of tricks – but the arrival of the novel coronavirus suddenly put her work at the center of a global crisis. “Never has my research been so relevant to the world,” she says.
The new virus, known as SARS 2, wasn’t a complete surprise, says Tiffany Tang, M.S. ’19, a fifth-year Ph.D. student in Daniel’s lab. She notes that new strains of coronavirus tend to emerge every decade or so, and the latest version was overdue. “When we heard there was a new virus in December, we figured it was about time,” she says. Of course, nobody could have anticipated the new virus’s devastating spread. “Part of me is still in shock over the impact,” she says.
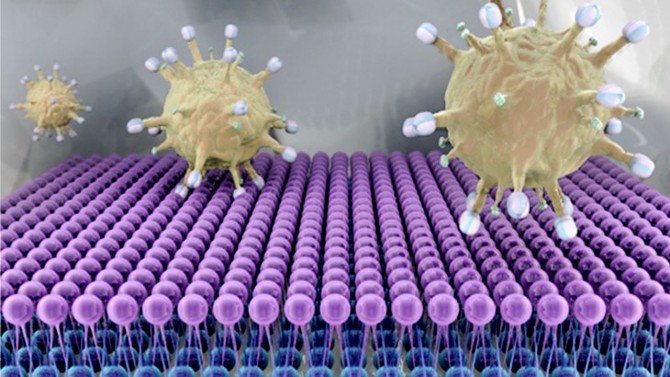
The novel coronavirus is far more contagious and deadly than its predecessor, but the two viruses also have much in common. The strains look alike under a microscope – picture tiny spheres bristling with spikes – and the similarities go deep down into the genome. By studying SARS 1, Daniel was learning much about unique features of coronaviruses that would help her understand SARS 2, even before it emerged.
“It was an easy pivot,” she says. “We could essentially deploy all of the techniques that we were already using.” With a new sense of urgency, Daniel and her team are collaborating with other Cornell researchers to expand their coronavirus studies in an effort that blends engineering with virology and data science.
“Understanding virus infection requires people of all different expertise coming together,” she says. “It’s truly an interdisciplinary effort.”
Discovering coronavirus’s vulnerability
Much of the work in Daniel’s lab looks at the pointy projections – called spike proteins – that give coronaviruses their tell-tale appearance. These spike proteins reach out to grab nearby cells (Daniel calls it “harpooning”), the first step toward an infection. Once the spikes have snagged a cell, the virus can deliver its genetic payload, essentially turning the infected cell into a virus factory. If there was a way to stop these spike proteins from doing their job, the virus would be essentially disarmed. “If the virus doesn’t fuse with the cell, the genome is never transferred and the infection doesn’t proceed,” she says.
Daniel and her team believe they may have identified a potential vulnerability in spike proteins. Studies of the original SARS 1 virus showed that the spikes depend on calcium to effectively do their job. As Daniel explains, calcium ions fit neatly within a particular region of the spike that controls the harpooning process. That extra fortification seems to help make the spike tips strong and rigid, qualities that are important for attaching to a cell. Without calcium, the spikes tend to flop, potentially making it more difficult for the virus to infect a cell. In theory, reducing calcium levels at the point of virus entry could stop infections before they start. Daniel and colleagues discussed the role of calcium in coronavirus infection in a paper published online April 6 in Antiviral Research.
When the new virus arrived on the scene, Cornell researchers knew where they wanted to look. Daniel – along with her frequent collaborators Gary Whittaker, professor of virology at the College of Veterinary Medicine, and Nicholas Abbott, the Tisch University Professor of chemical and biomolecular engineering in the College of Engineering – received a $200,000 “rapid” grant from the National Science Foundation in April to study the spike proteins of the new virus. The team brings together different skills and viewpoints: Whittaker’s lab had been studying the spike proteins in coronaviruses, including varieties that infect dogs, cats, pigs and horses; Abbott’s lab studies hydrophobic interactions, which coronaviruses use to harpoon cells.
The researchers are paying particularly close attention to the section of the protein, called the fusion peptide, that initiates a process called membrane fusion, where the host and viral surfaces merge, forming an opening where the virus’s genome escapes into the cell. After comparing the sequences for the fusion peptides in SARS 1 and SARS 2, they quickly realized that this novel threat was surprisingly familiar. “They are nearly an identical match,” Daniel says. “And because we knew that calcium was crucial for SARS 1 entry, we posited that SARS 2 would require it, too.”
If the new coronavirus really does need calcium to infect a cell, an arsenal of calcium-blocking drugs approved by the Food and Drug Administration could potentially be called into action. The drugs, often prescribed to treat high blood pressure and heart problems, work by reducing the concentration of calcium ions around the cell. If Daniel’s theory about calcium and spike proteins is correct, those drugs at the right concentrations in the right patients could slow or even prevent infection of the novel coronavirus.
As a first test of the hypothesis, Daniel and her team have been studying the effects of calcium-blocking drugs on lung cells and kidney cells that are exposed to the new coronavirus in the laboratory. The findings are not yet published, but Daniel is already excited. “We’ve had phenomenal results,” she says. “In some cases, we’ve had undetectable levels of infection.”
Turning to big data, nature
Daniel is quick to point out that there’s a huge gulf between showing that drugs work in a petri dish and showing that they work in patients. She cautions that it’s far too soon for people to take such medicines to fight or prevent coronavirus infection.
As a next step, she’s hoping to build on ongoing collaborations with other Cornell researchers to test the theory in more realistic models. “I would love to team up with researchers in the clinic and veterinary school to see how this treatment works in mice and guinea pigs,” she says.
There may be another way to test the possible effects of calcium-blocking drugs on COVID-19 infection. Daniel notes that many people already take such drugs for other conditions. By mining data in medical records, it might be possible to find out of if those patients are any less likely than others to succumb to the infection. The comparison would be complicated by the fact that people taking the drugs are by definition already somewhat unhealthy.
But if there’s any benefit to calcium-blocking drugs, the numbers should tell the story.
“We’ll need to turn to someone with the right computational toolbox to tease that out,” she says, “Luckily, we know some great people down at Weill Cornell Medicine.”
In another line of investigation, Daniel and team are looking at the interaction between spike proteins and antibodies produced by the immune system. So far, they’ve managed to show that mere fragments of the spike protein were enough to trigger the production of antibodies in mice.
What’s more, some of those antibodies seemed to zone in on the fusion peptides of invading viruses. The immune system seems to be targeting the same parts of the virus that scientists have in their own sights. Understanding how nature fends off coronaviruses could offer important insights for researchers trying to accomplish the same goal, Daniel says.
After building her career on bioengineering techniques, Daniel had to move slightly beyond her comfort zone to a more clinical world of cell cultures and animal studies. She embraced the challenge.
“If I were to focus solely on the technology that I developed, I’d be missing the bigger picture,” she says. “As an engineer, my role is bringing my toolbox to the field of virology, and then to help synthesize the information from all inputs to better understand these viruses.”
This article is adapted from “Biomolecular weapons for fighting COVID-19” by Chris Woolston, a freelance writer for the College of Engineering. The article appeared in the fall 2020 issue of Cornell Engineering magazine.
Media Contact
Get Cornell news delivered right to your inbox.
Subscribe