Ultrawide bandgap gives material high-power potential
By David Nutt
The path that leads to the next generation of high-power electronics is not long. But it needs to be wide. Very wide.
A Cornell collaboration has found a way to grow a single crystalline layer of alpha-aluminum gallium oxide that has the widest energy bandgap to date – a discovery that clears the way for new semiconductors that will handle higher voltages, higher power densities and higher frequencies than previously seen.
The team’s paper, “Crystal Orientation Dictated Epitaxy of Ultrawide-Bandgap 5.4- to 8.6-eV alpha-(AlGa)2O3 on M-Plane Sapphire,” published Jan. 8 in Science Advances. The co-lead authors are Riena Jinno, a former visiting researcher, and Celesta Chang, Ph.D. ’20.
The paper is the latest finding of the Air Force Research Laboratory-Cornell Center for Epitaxial Solutions (ACCESS) lab, which launched in 2018 with the goal of exploring the potential uses of gallium oxide for next-generation electronics.
The collaboration was led by co-senior authors Debdeep Jena and Huili Grace Xing, both professors in electrical and computer engineering and in materials science and engineering. The team also included David Muller, the Samuel B. Eckert Professor in Applied and Engineering Physics, who specializes in electron microscopy, and Darrell Schlom, the Herbert Fisk Johnson Professor of Industrial Chemistry, who grows oxide materials for electronic uses.
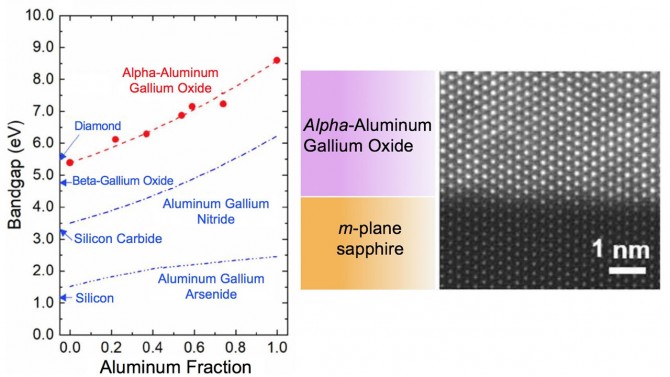
The gallium oxide family comes in a variety of flavors, or phases, that share the same atoms but in slightly different configurations that generate a whole different slate of electronic and optical properties. Scientists have so far favored the beta phase because it’s the most stable form of the material. But the phase that has the most tantalizing prospects, due to its wider bandgap, is alpha-gallium oxide and its alloys with alpha-aluminum oxide, commonly known as sapphire. The main drawback to the alpha phase gallium oxide is that it isn’t as stable as its beta sibling.
The researchers set about creating a more stable alpha-gallium oxide by growing it on a substrate of sapphire, which has a similar atomic structure. Jinno used molecular beam epitaxy – known informally as “atomic spray-painting” – to create the single layer film. Chang then used scanning transmission electron microscopy to study how its atoms were arranged and detect any defects that may have been created by crystal lattices that weren’t perfectly aligned. Those very defects often give materials unique characteristics, but they can sabotage the performance of electronic or photonic devices.
One of the issues Jinno and Chang encountered was that different phases sometimes crept into their samples.
“Sapphire has a lot of facets,” Chang said. “If you grow on one facet, and then on the other facet, the quality of the alpha-gallium oxide films will be totally different. It doesn’t always result in a good, clean, quality film.”
The team eventually identified the right symmetry of the right sapphire facet, called the m-plane, which resulted in a stable alpha-gallium oxide film. They slowly replaced some of the gallium atoms with aluminum as a way to widen its bandgap even more.
Conventional gallium oxide has a bandgap of 3 to 4.7 eV (electron volts); every electron volt represents a huge leap in performance. Beta-gallium oxide reaches up to 4.8 eV. The bandgap of the new alpha-aluminum gallium oxide starts at 5.4 eV and, as more aluminum is swapped in, it expands to 8.6 eV – almost eight times the bandgap of silicon.
“These are actually the widest energy bandgap crystals that have ever been realized by epitaxy. The fact that Riena and Celesta could grow and understand these atomic layers and found the right sort of playground – which is the surface of sapphire – to grow them on is a major breakthrough,” said Jena, who connected the current work to the discovery of high-quality crystalline gallium nitride, which over the last two decades has revolutionized LED lighting.
Not only is alpha-aluminum gallium oxide robust enough to handle enormous amounts of energy at high speeds and high temperatures, it is also lightweight and compact – qualities that could make it a crucial component in aeronautical technologies as well as other high-power electronics.
The material is also incredibly efficient, which could reduce the loss of energy in converting and transmitting solar power.
“This is really moving semiconductors into unchartered territory in terms of how much energy they can handle,” Jena said. “At the same time, it is forcing us to rethink the materials that we always believed to be insulators, like sapphire. Can we actually change their properties to our own benefit in terms of controlling their energy gaps and electronic properties by doping it as we do with silicon and gallium nitride?”
Co-authors include Takeyoshi Onuma of Kogakuin University, Japan; research associate Yongjin Cho; Michael Cao, Ph.D. ‘20, Kevin Lee, Ph.D. ‘20, Shao-Ting Ho, M.S. ’20 and Derek Rowe, M.S. ’20; and senior research associate Vladimir Protasenko.
The research was supported by ACCESS, the JSPS Overseas Challenge Program for Young Researchers and the Air Force Office of Scientific Research.
Media Contact
Get Cornell news delivered right to your inbox.
Subscribe