Shaoyi Jiang: protective materials that mimic water
By Jackie Swift
Shaoyi Jiang, Ph.D. ’93, is the Robert S. Langer ’70 Family and Friends Professor in the Meinig School of Biomedical Engineering. His research focuses on the molecular understanding, design and development of functional zwitterionic materials for biomedical and engineering applications.
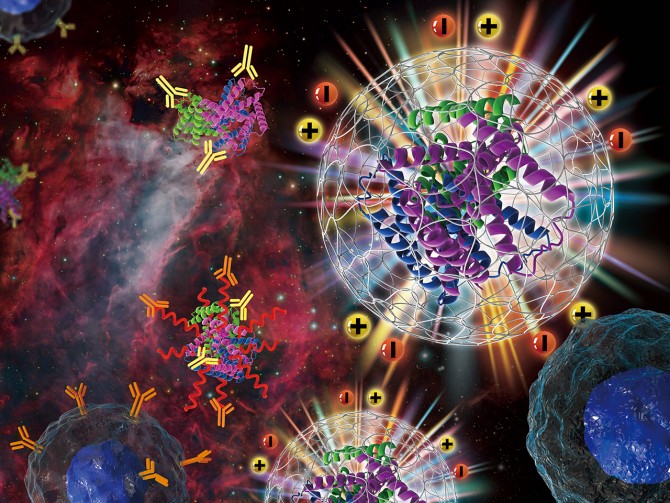
Biocompatibility is an enduring challenge for medical applications. Our immune systems are adept at identifying foreign bodies, then rendering them harmless: encapsulating them in collagen to isolate them, attempting to dissolve them with enzymes, or generating antibodies to kill them. The trouble is, our bodies see every intruder as an enemy – whether it’s a dangerous virus or a life-saving medical implant.
This problem has come to the fore recently in the development of the groundbreaking mRNA vaccines for COVID-19, explains Shaoyi Jiang, professor of biomedical engineering. These vaccines feature polyethylene glycol (PEG) as a stabilizing coating around the mRNA cargo, preventing it from being destroyed by the body before it can stimulate the production of COVID-19 antibodies.
Occasionally a patient may have an allergic reaction to one of the vaccines, possibly due to overexposure to PEG, which is present in shampoos, drugs, toothpaste and other products.
“For about 45 years now people have been using PEG in all kinds of products because it is considered hydrophilic [water-loving],” says Jiang, who joined the Cornell faculty in 2020. “In medical applications, it is used to protect drugs in the bloodstream and also implants in the body. Unfortunately, our bodies see it as a foreign material and attack it, as it is not as hydrophilic as one would think.”
Zwitterionic: fooling the immune system
PEG and the question of biocompatibility have been central to Jiang’s research for the past 20 years and led to his invention of a new class of materials, known as zwitterionic.
Zwitterionic materials are hybrids, carrying one positive and one negative ion, which means they are extremely hydrophilic while neutral in net charge.
“A zwitterionic material looks just like water,” Jiang says. “That’s why you can put it on the surface of a medical implant or use it to replace PEG as protection for a drug, and our immune systems won’t attack it.”
In 2013, Jiang and his collaborators excited the biomedical world when they published a paper in Nature Biotechnology showing that implants coated in their zwitterionic material lasted three months without encapsulation in animal models. Until that breakthrough, the standard timeline for non-encapsulation was one month.
“In 2016, Nature Biotechnology chose our work as one of eight major biomedical research ‘hits’ published in the journal over the previous 20 years,” says Jiang. “A reporter from the magazine asked me my future plans, and I told her I was going for the next milestone because we only showed three months without encapsulation and I thought we could go further than that.”
In January, Jiang and his co-authors published another paper in Science Advances, showing no encapsulation of zwitterionic materials implanted in animal models for up to one year. Those findings are the culmination of years of work to prove the concept of zwitterionic material as biocompatible.
Nontoxic marine coatings
While working on the biocompatibility question, Jiang also turned his attention to another application of his new materials: environmentally benign marine coatings to prevent the buildup of fouling organisms on ship hulls.
“Nature is very smart, so if a material works on a particular species – for example, algae – then it doesn’t work for some other species, such as diatoms (single-celled algae),” he says. “When we first started testing our zwitterionic material and nothing stuck to it, people said, ‘I’ve been trying this for years, and I’ve never seen anything like this before. Why does your material work for every organism?’”
The other researchers thought the answer must be that Jiang’s material is highly toxic. “That’s because 90% of marine coating products today keep hulls free of fouling organisms by releasing heavy metal ions and organic biocides that kill anything that tries to stick to them,” he says.
Zwitterionic material, however, is nontoxic and works by coating the ship hull in a layer of water, essentially making it invisible to marine organisms. They don’t stick to it because they don’t recognize it, similar to how the material fools the body into ignoring implants, Jiang says. His zwitterionic hull material will be tested soon on U.S. Navy ships.
Real-world applications
Over the years, Jiang has looked at ways to commercialize some of the new zwitterionic technologies he invented. He holds 55 patents and founded or co-founded three startups based on his research. Now he is beginning a new phase of his career at Cornell with a two-pronged research approach that should result in more real-world innovations: one stream of research focusing on the fundamental understanding of biocompatibility and the other on translational applications for zwitterionic materials.
“When people have asked why my material works so well, why it works for drugs and implants and antifouling, I have always said that biological systems see it as water,” Jiang says. “But now I want to upgrade our science. My group is starting to work with people in immunology, for instance, to study how our entire immune system responds to zwitterionic material. Only when we understand all the details of the body’s response can we define what biocompatibility really is.”
The current mode for testing biocompatibility is to implant a device and wait a month to see if the body rejects it. If not, it is considered biocompatible, Jiang explains.
“I want to know what exactly happens inside the body,” he says. “My ideal is to get people from different disciplines to work together to deal with this fundamental issue.”
On the translational side, Jiang and his lab have begun collaborations with several groups at Weill Cornell Medicine. His ultimate goal is to explore the application of zwitterionic material for a wide range of medical needs, including regenerative medicine, stem cell technology, tissue engineering, nanomedicine, cancer immunotherapy and vaccines.
“Cornell offers the chance to collaborate with so many people,” he says. “I’ve talked to many researchers on the Ithaca campus and at Weill Cornell, and they’ve all been really interested in working together on this.”
Innovation at the molecular level
When he started out, Jiang was a computational chemist; biomedical engineering was not where he expected to end up. His Ph.D. was in chemical engineering, and his early training mainly focused on molecular simulation and modeling, statistical mechanics and quantum chemical calculations. Because of that, he was able to look at the issue of biocompatibility differently from most other researchers.
“I use the molecular view to look at how a material works,” he says. “For example, in 2000 I became interested in why PEG works as well as it does. So I used my previous training in computational, molecular-level understanding to study the mechanism behind PEG. When we finally realized how it works, then immediately we knew why it doesn’t work as well as we need it to.
“We were able to think outside the box,” he said, “and come up with a new class of materials to take its place based on the fundamental principles we learned.”
Jackie Swift is a freelance writer for Cornell Research, where this story originally appeared.
Media Contact
Get Cornell news delivered right to your inbox.
Subscribe