News directly from Cornell's colleges and centers
Giometto to study evolution of microbial populations with $1.9M grant
By Chris Dawson
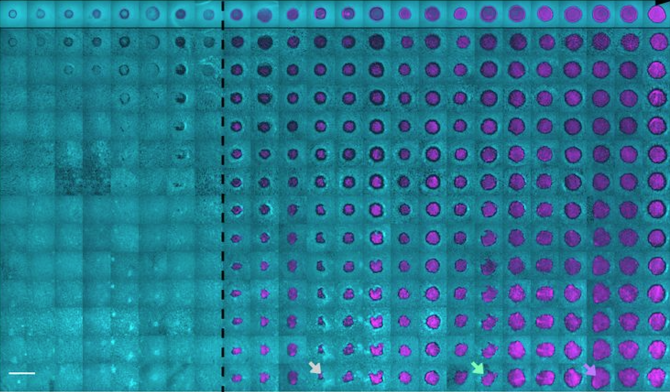
Much of what is currently known about the evolutionary dynamics of microbes that have an impact on human health or the environment comes from studies carried out in lab cultures that exist in well-mixed liquids. A new Cornell research project aims to gain a better understanding of how these same microbes interact on surface environments, such as human skin, where their dynamics are less understood.
The project, “Evolutionary Dynamics of Dense, Spatially Structured, and Antagonistic Microbial Populations,” is being led by Andrea Giometto, assistant professor in Cornell’s School of Civil and Environmental Engineering, using a $1.9 million Maximizing Investigators’ Research Award from the National Institute of General Medical Sciences.
Giometto relies on statistical and nonlinear physics methods, experiments with microbes, and genetic engineering to examine the spatial growth of microbial communities. Much of his research is fundamental, with the hope of understanding how ecological and evolutionary processes play out in spatially extended populations.
In some of his earlier published work, Giometto showed that a strong toxin-producing microbe can invade a population of a weaker toxin-producing microbe only if the initial size of the invading microbe population – the inoculum – is sufficiently large. If the inoculum is too small, it eventually disappears even if its toxin production is stronger. The new, five-year, grant will fund the next logical steps in Giometto’s efforts to understand the population dynamics of antagonistic microbial populations.
“It is a wonderful award,” Giometto said. “It is designed to give early-stage investigators a guaranteed consistent source of support at the beginning of our careers as principal investigators. It is a real vote of confidence in our work and our approach.”
Giometto added that understanding how antagonistic microbial populations – such as those that occur in the human gut and on the skin – interact is an essential step to understanding the complex relationship between humans and their microbiome.
Giometto believes that where a microbe is located can strongly affect how it evolves. In experiments with microbes growing on surfaces, he found that mutations that confer resistance to toxins released by competitors are selected for at the interface between the competitors. In an ongoing experiment, yeast cells selected at the expanding edge of colonies are evolving changes in cell shape, becoming more and more elongated as the experiment progresses.
To fill in gaps in what is understood about how microbes behave on surfaces, Giometto will use the grant to carry out studies to quantify how spatial structure, mechanics and biological interactions impact the adaptive evolutionary dynamics of microbial populations. He then hopes to take these studies a step further by investigating how antagonistic interactions among microbes affect the dynamics of invasion in the gut of a species of nematode.
“These experiments will help us translate results obtained in simple laboratory settings to the more complicated but more realistic dynamics of invasion of a host microbiome,” Giometto said.
Media Contact
Get Cornell news delivered right to your inbox.
Subscribe