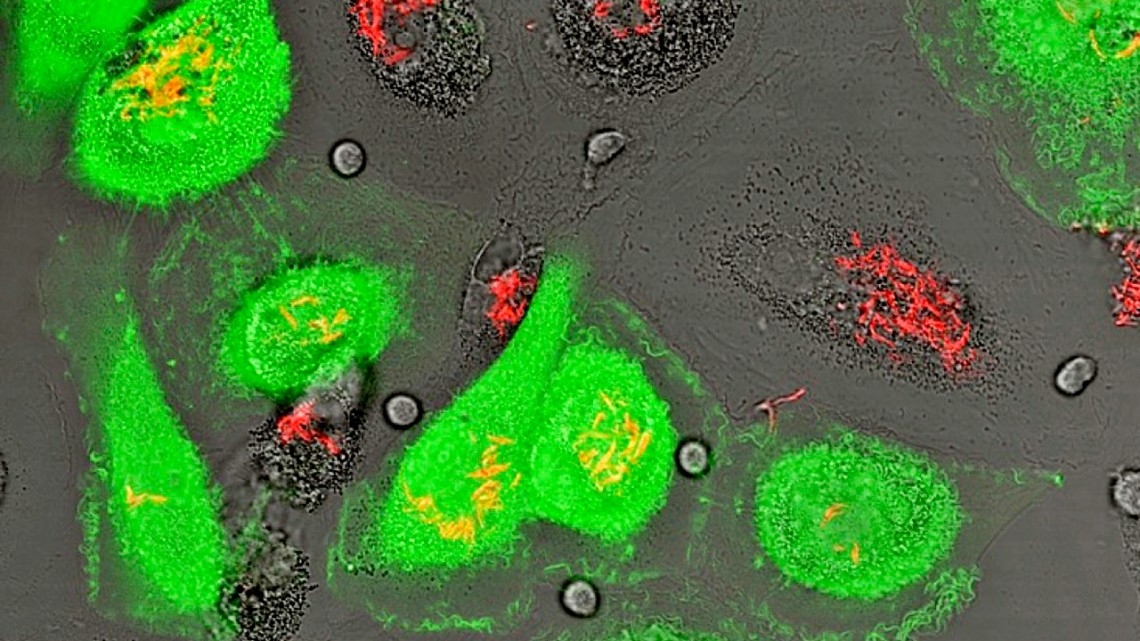
The fluorescence micrograph shows human macrophages that are experimentally infected with both HIV-1 (that expresses green fluorescent protein) and Mycobacterium tuberculosis (that expresses mCherry fluorescent protein). Macrophages support the growth of both infectious agents.
News directly from Cornell's colleges and centers
$3.4M grant to tackle ‘biggest challenge’ to HIV cure
By Krishna Ramanujan
Cornell researchers will use a five-year, $3.4 million grant from the National Institutes of Health to investigate whether chemical inhibitors of epigenetic regulation – including many FDA-approved drugs – could be re-purposed to treat HIV-1 infections that are persistent in tissues and represent the biggest challenge for a cure.
Most research on HIV-1 focuses on an immune cell type called CD4 T lymphocytes, which coordinate an immune response in the blood by stimulating other immune cells, including macrophages, to fight infection.
Lymphocytes, when infected with HIV-1, are driven to cell death. When combined with anti-HIV drugs that suppress new infections by limiting the virus’s ability to replicate, these treatments are effective in eliminating HIV-1 from lymphocytes.
Unfortunately, the treatment doesn’t affect HIV-1 reservoirs found in infected macrophages that reside in tissues. Though more research is needed, it’s been known for some time that macrophages – immune cells known for engulfing microorganisms and pathogens – also become infected with HIV-1.
“I think the HIV community will agree that the biggest challenge towards curing HIV-1 is getting rid of persistent viral reservoirs,” said David Russell, professor of microbiology and immunology at the College of Veterinary Medicine and principal investigator of the grant.
Macrophages are present in both the blood and in tissues. Tissue resident macrophages, such as alveolar macrophages found in the lungs and microglial cells in the brain, come from fetal stem cell lineages that populate tissues when embryos are developing.
Russell and grant collaborator Henry Mwandumba, professor of medicine at Kamuzu University of Health Sciences, and director of the Malawi-Liverpool-Wellcome Programme, have identified HIV-1 reservoirs in alveolar macrophage populations in the lungs of infected donors. They found that alveolar macrophages with latent HIV-1 infections did not die off when infected, as lymphocytes do, and show differences in their epigenetic regulation when compared to macrophages in the blood.
In the grant, Russell and colleagues plan to use a library of 735 known epigenetic inhibitors – 70 of which are already FDA-approved – to identify host cell regulatory pathways that impact HIV-1 persistence. The goal is to identify compounds that selectively drive cell death in HIV-1 infected macrophages. Then existing antiretroviral therapeutics can be used in combination to prevent new infections. Previous work in the Russell lab had shown that HIV-1 infection of macrophages induced pro-survival pathways, which they hope to target with these inhibitors.
The researchers will conduct transcriptional profiling and compare alveolar macrophages and blood-derived macrophages to assess the cellular responses to HIV-1 infection in the two lineages. Candidate compounds will be evaluated for their ability to drive cell death and reduce viral reservoirs in tissue culture with alveolar macrophages isolated from HIV-1 positive human donors in Malawi.
The persistence of HIV-1 in the lung is of particular significance as a third of deaths of people living with HIV are due to co-infections from tuberculosis (TB), and in Malawi approximately 80% of TB cases are HIV-1 positive. Mwandumba, who has collaborated with Russell for the last two decades on both HIV-1 and tuberculosis infections, was named Cornell’s Distinguished African Scholar in 2015.
Media Contact
Get Cornell news delivered right to your inbox.
Subscribe