Tiny networks intertwine to mimic design of bird colors
By David Nutt, Cornell Chronicle
The bright plumage of birds is often a feast for the eyes, but it has been a headache for scientists who have struggled to recreate the photonic nanostructures that generate those colors in the lab.
Part of the challenge is developing structures at the awkward scale of a few hundred nanometers: too big for molecular chemistry, yet too small for direct fabrication.
A team led by Eric Dufresne, a professor with joint appointments in the Department of Materials Science and Engineering in Cornell Engineering and the Department of Physics in the College of Arts and Sciences, has developed a method to efficiently engineer these intricate nanostructures through a form of phase separation – a process akin to the way water and oil uncouple in salad dressing.
The resulting materials could prove useful in a variety of applications, from making sustainable pigments to energy storage and filtration.
The team’s paper, “Elastic Microphase Separation Produces Robust Bicontinuous Materials,” published Oct. 26 in Nature Materials. The lead author is Carla Fernández-Rico, a postdoctoral researcher at ETH Zurich.
For years, Dufresne has found inspiration in the natural world. By studying the inner workings of living systems such as birds and insects, he seeks to uncover new physical mechanisms that could inform the design of functional synthetic materials.
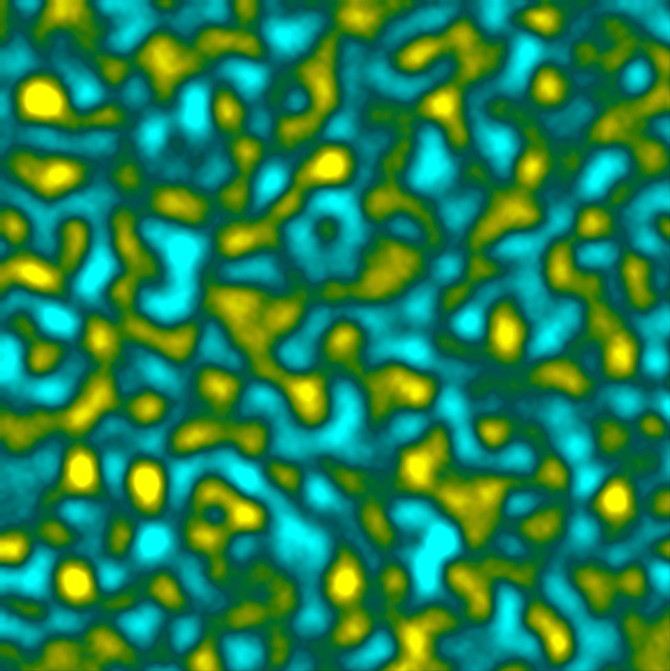
For their latest project, Dufresne’s team set out to create a “bicontinuous” material, which he describes as containing two “crazy interpenetrating networks” – rubber and oil – that are perfectly intertwined in a precisely defined structure, yet never sacrifice their own identity or characteristics.
“In a sponge, fluid and solid are interwoven,” Dufresne said. “Together, they can do more than the sum of their parts. Bringing together two materials in a similar way at the nanoscale can unlock new functionalities, but presents all sorts of challenges.”
In the past, materials scientists focused on two approaches to make bicontinuous nanostructures: self-assembly and phase separation.
“You either start with building blocks at the size you’re looking for and assemble them. Or you take a mix of molecules that don’t like each other, like oil and water. They just separate on their own, but it is hard to control the sizes of the structures they make,” Dufresne said. “We wanted to have all the control that you get with the assembly method, but to keep the simplicity and low cost of the separation method.”
In their new paper, Dufresne’s team introduce a strategy called Elastic MicroPhase Separation (EMPS). The initial experiment was decidedly low-tech. They submerged a piece of silicone rubber – i.e., “the elastic matrix” – in a bath of fluorinated oil – essentially liquid Teflon – and heated it in an oven at 60 degrees Celsius. Once the oil had been absorbed by the rubber after a few days, the researchers let it cool to room temperature.
“At room temperature, the oil and rubber don’t like to be in the same place. And they make this amazingly intricate structure,” Dufresne said. “Hosting the separation process inside of rubber prevents the separated oil from making one big lump, like in salad dressing.”
The real challenge was measuring and interpreting their results. The nanostructures were barely visible in a normal light microscope, yet the material was too “squishy” for an electron microscope. The team turned to 3-D fluorescence microscopy, which revealed they had successfully created a bicontinuous material at the desired size.
While the researchers are excited by the possibilities of their new approach, they still aren’t really sure how it works.
“We can give a bunch of reasons why it shouldn’t have worked, but it worked,” Dufresne said. “That’s why it’s not just an exciting engineering contribution, it’s also an exciting physics thing, because we really don’t know what the actual mechanism is. We know we can get a range of different types of structures, which we can tune by changing the different types of silicone rubber. So we’re trying to understand why that is and what its limitations are. Can we make things much smaller? Much bigger? This was really just a proof of concept. Now we want to use the same ideas to structure a broader range of materials for potentially useful applications.”
Co-authors include researchers from ETH Zurich.
The research was supported by the ETH Zurich Fellowship and the Swiss National Science Foundation’s National Centres of Competence in Research for Bioinspired Materials.
Media Contact
Get Cornell news delivered right to your inbox.
Subscribe