Gut muscle vital for absorbing fats forms like scars
By Krishna Ramanujan, Cornell Chronicle
By discovering how a type of smooth muscle – which is essential for mechanical aspects of absorbing fats from food – forms in the gut, Cornell scientists have opened doors to making artificial muscle, repairing muscle following gut surgeries and treating inflammatory bowel disease and obesity.
The findings, published online March 26 in a study in Developmental Cell, reveal that intestinal smooth muscle originates in embryos and forms by the same process that is a hallmark of creating scar tissue when a wound heals.
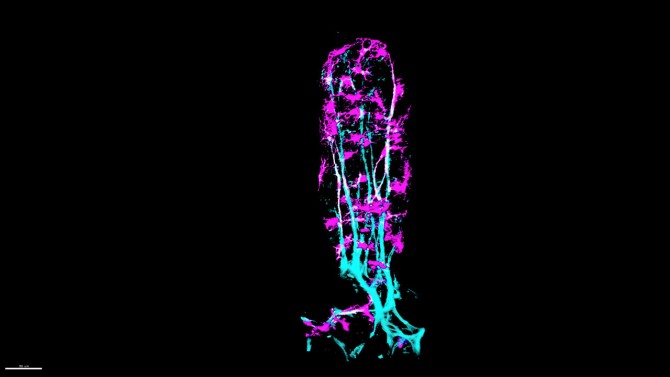
The smooth muscle sits inside tiny finger-like projections called villi, which absorb fats – also known as lipids – from foods. Contractions of these smooth muscles squeeze absorbed dietary fats through lymphatic capillaries, called lacteals, which send the fats into the systemic blood circulation for producing energy.
Despite this vital function, until now it’s been unknown how these muscles form, assemble alongside lacteals and are maintained.
“We have discovered the origin of lymphatic smooth muscle that is critical for lipid absorption and transport,” said Natasza Kurpios, professor of molecular medicine in the College of Veterinary Medicine and co-corresponding author of the study, along with Iwijn De Vlaminck, associate professor of biomedical engineering at Cornell Engineering.
Bhargav Sanketi, Ph.D. ’23, a former member of Kurpios’ lab, and Madhav Mantri, Ph.D. ’23, a former member of De Vlaminck’s lab, are both co-first authors of the paper. The two students were previously roommates, and came up with the idea for the study and linking the two labs’ expertise together over a casual tea.
De Vlaminck’s lab specializes in state of the art transcriptomics: RNA sequencing that reveals gene expression, or which genes are turned on and off. His lab ran single-cell transcriptomics to track where and how intestinal smooth muscle develops and followed the process through every stage of development.
“We reconstructed, based on gene expression, where this very unique muscle comes from,” Kurpios said. Intestinal smooth muscle originates from a mesenchymal cell, a kind of stem cell. “Our paper is the first one to generate an atlas of the intestinal mesenchyme at all the key embryonic stages of intestinal development,” she said.
The atlas allowed the researchers to identify specialized mesenchymal stem cells called fibroblasts that kick off the process of creating intestinal smooth muscle. These cells divide quickly and give rise to muscle cells, but first they go through an intermediate stage, or a myofibroblast, which starts contracting and then differentiates into different types of muscle.
“One reason why this was so exciting is that this fibroblast to myofibroblast transition is the same process that initiates wound healing,” Kurpios said. When people cut their fingers, for example, fibroblasts are recruited and transition to myofibroblasts, which contract and bring two sides of the cut together to make a scar.
By understanding the process, Kurpios believes there is a possibility to develop drugs for growing artificial muscle or repairing muscle following gut surgeries. The single-cell technology has revealed all the genes that are differentially expressed along this transition from fibroblast to myofibroblast, which researchers who study scarring may also now explore, she said. The findings could also point to a treatment for fibrosis, when scarring doesn’t stop when it should, causing an overgrowth and hardening of scar tissue.
Finally, the researchers found a pathway that governs proper alignment of intestinal smooth muscle with neighboring lacteal cells, which is necessary for moving fats into the blood. For this pathway to activate, the scientists found the two types of cells must come into physical contact. In experiments, mutant mice whose muscle cells failed to make contact with lacteal cells could not absorb fats.
The study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institutes of Health (NIH), the Cornell University Center of Vertebrate Genomics Scholarship, the Cornell University Center of Vertebrate Genomics Seed Grant, the Cornell University College of Veterinary Medicine Graduate Research Fellowship, and the NIH for the Cornell Institute of Biotechnology.
Media Contact
Get Cornell news delivered right to your inbox.
Subscribe