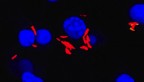
David Russell, the William Kaplan Professor of Infection Biology.
Scientists find new targets in the war against tuberculosis
By Lauren Cahoon Roberts
A new study by Cornell College of Veterinary Medicine scientists unveils a novel approach to vaccine development in the fight against tuberculosis, illustrating how certain host cells are able to either control or promote the growth of the Mycobacterium tuberculosis (Mtb) bacterium.
Ninety to 95 percent of people infected with tuberculosis (TB) live with the bacteria in their systems without manifesting symptoms. For that minority in which the disease takes hold, however, tuberculosis is often deadly. David Russell, senior author on the paper in the Journal of Experimental Medicine and the William Kaplan Professor of Infection Biology, said the immunological understanding for why some people’s disease progresses is still unclear.
“We’ve asked the questions the wrong way,” Russell said. “How do you design a vaccine when you have no idea what protective immunity looks like?”
Previous vaccines followed a traditional paradigm: Challenge the body so that it produces an adaptive immune response, usually indicated by the presence of certain cytokines, immunological indicators of adaptive immunity. Yet, in all vaccine trials to date, individuals given these vaccines have not developed protection.
Russell sidestepped the traditional approach of only using immunological indicators as signs of protection against Mtb, and expanded the view to include the pathogen itself. To do this, his team developed bacterial fitness reporters – fluorescent Mtb bacteria that can easily be seen and analyzed within cells. Russell first examined how these glowing bacteria behaved in controlled in vitro and in vivo conditions.
Macrophages are the primary cells that TB bacteria infect in the lung. The lung has two kinds of macrophages: alveolar macrophages (AM) and interstitial macrophages (IM). Using his fluorescent bacteria, Russell found that the AM cells in infected mice supported the growth of higher numbers of Mtb bacteria than did the IM cells. However, in infected mice whose AM cells were depleted, their bacterial load decreased by more than 80 percent. The AM cells were permissive, while the IM cells were protective.
Russell believes the reason for such a dramatic difference between these macrophage types could be nutrient availability. When stressed with infection, IM cells rely on glucose for energy. AMs turn to fatty acids instead – consuming molecules like cholesterol, which is a preferred source of nutrient for Mtb bacteria in the host.
This finding could lead the way to host-directed therapeutics that would target permissive host cells like the alveolar macrophages, or target cholesterol metabolism to try to starve the TB bacteria—a concept know as nutritional immunity. Russell said these new findings lay groundwork for a more pragmatic approach to tuberculosis therapeutics where controlling the bacteria, rather than exterminating it, is the goal.
“Killing the bacteria may not be as important as people assume,” Russell said. “Growth restriction could prove to be more effective.”
This insight into permissive and protective macrophages will help Russell as he screens millions of possible compounds as part of a $3.1 million drug discovery grant from the Gates Foundation. So far, the Russell lab has screened 1.5 million compounds for possible anti-TB properties.
“We have already found that the environment within the host cell restricts bacterial metabolism, and reveals new drug targets not found in bacteria in broth culture,” says Russell.
Armed with the latest information on the macrophage hosts, the Russell team can make even greater headway in their hunt for a viable cure for TB.
Lauren Cahoon Roberts is assistant director of communications at the College of Veterinary Medicine.
Media Contact
Get Cornell news delivered right to your inbox.
Subscribe