Better implant device may ease therapy for Type 1 diabetes
By Jenny Stockdale
A Cornell-led research team’s improved cell therapy device effectively secreted insulin and controlled blood sugar in diabetic mice for up to six months – showing promise for the possibility of an effective, complication-free treatment for Type 1 diabetes, a chronic disease with no known cure.
The research group, led by associate professor Minglin Ma from the Department of Biological and Environmental Engineering in the College of Agriculture and Life Sciences, partnered with stem cell biologists at Washington University School of Medicine in St. Louis to identify how to protect these islets to let them do their job safely.
Cornell doctoral student Xi Wang is the first author of the group’s paper, “A Nanofibrous Encapsulation Device For Safe Delivery of Insulin-producing Cells to Treat Type 1 Diabetes,” published June 2 in Science Translational Medicine. Postdoctoral researcher Daniel T. Bowers also contributed to the report.
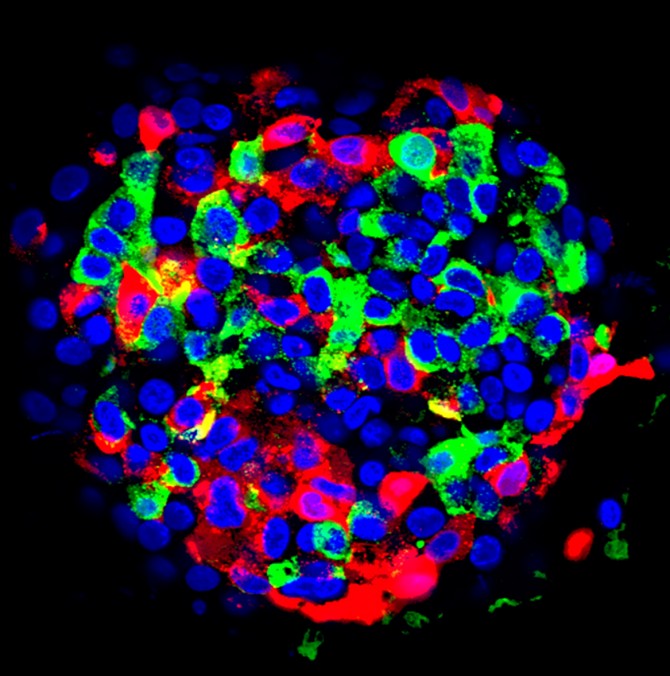
Type 1 diabetes – which afflicts roughly 1.6 million Americans, including nearly 200,000 children and adolescents – causes insulin-producing pancreatic cell clusters (islets) to be destroyed by the body’s own immune system. Past research has shown that islets can be grown from stem cells and implanted into the body, but they need to be able to secrete insulin in response to blood sugar while also being protected from the body’s immune and autoimmune responses.
Ma’s team implanted a device containing islets or human stem-cell derived, insulin-secreting cells into diabetic mice, reversing their diabetes. By doing so, they eliminated the need for drugs to suppress the immune system from attacking islets.
“The combined structural, mechanical and chemical properties of the device we used kept other cells in the mice from completely isolating the implant and, essentially, choking it off and making it ineffective,” Ma said. “The implants floated freely inside the animals, and when we removed them after about six months, the insulin-secreting cells inside the implants were still functioning. And importantly, it is a very robust and safe device.”
Think of the device as a tiny, micro-porous cage, about the width of several strands of hair, that gets implanted into the abdomen. The mesh of the cage safely encloses the islets to secrete insulin in response to blood sugar levels and gives them a steady flow of nutrients and oxygen to keep them alive, but protects them from the immune cells that are too big to get through.
Several implants have been tried in recent years, with varying levels of success, including earlier devices made by Ma’s research team in 2018 that employed a similar, but less sturdy design. For this study, Ma and his colleagues developed what they call a nanofiber-integrated cell encapsulation (NICE) device – a safer, more effective method. They filled the implants with either islets or insulin-secreting beta cells that had been manufactured from stem cells, then implanted the devices into the abdomens of mice with diabetes.
“We’d rather not have to suppress someone’s immune system with drugs because that would make the patient vulnerable to infections,” said Jeffrey Millman, an associate professor of medicine at Washington University and one of the study’s co-senior investigators. “The device we used in these experiments protected the implanted cells from the mice’s immune systems, and we believe similar devices could work the same way in people with insulin-dependent diabetes.”
The cells in the implants continued to secrete insulin and control blood sugar in the mice for up to 200 days. And those cells continued to function in spite of the fact that the mice were not treated with anything to suppress their immune systems.
“This project is such a wonderful example of what scientific collaboration is all about,” said Dr. James Flanders, an associate professor emeritus in the College of Veterinary Medicine and collaborator on the project. “It represents several years of integrated, focused work involving investigators from several departments and universities. It’s been a tremendously rewarding experience working with Dr. Ma and his team as we’ve striven to perfect the device to achieve our current success in mice as well as in dogs.”
Additional research will be needed to scale up this treatment for human application, as well as to determine how fibrosis – wound healing that can cause scar tissue development around the device and choke the islet cells – could be further mitigated.
This work was supported by Novo Nordisk, the Hartwell Foundation and the Juvenile Diabetes Research Foundation. Additional funding was provided by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health.
Jenny Stockdale is associate director of marketing and communications in the College of Agriculture and Life Sciences.
Media Contact
Get Cornell news delivered right to your inbox.
Subscribe