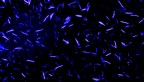
Lipid expansion microscopy uses the ‘power of click chemistry’
By Kate Blackwood
Lipids – fats – make great walls for cells and organelles because they are water resistant and dynamic. But those same characteristics also make them hard to image using expansion microscopy, a technique that works for magnifying other cell components.
Researchers in the lab of Jeremy Baskin, associate professor of chemistry and chemical biology in the College of Arts and Sciences and member of the Weill Institute for Cell and Molecular Biology, have discovered a way to apply expansion microscopy, which expands cell components to make them more visible, to lipids using click chemistry, recognized with the 2022 Nobel Prize in Chemistry.
“Lipid Expansion Microscopy” published in the Journal of the American Chemical Society on October 3, a few days before Baskin’s related doctoral research appeared in the chemistry Nobel Prize announcement.
Lipid Expansion Microscopy (LExM) enables closer study of biological membranes, the site of critical cell signaling and nutrient exchange, said Brittany White-Mathieu, National Institutes of Health Postdoctoral Fellow in the Baskin Lab and first author of the paper.
“If these processes are disrupted, it can lead to disease,” White-Mathieu said. “Being able to image membrane interactions or just how membranes change when they are introduced to certain stimuli can shed light on how cells respond to external things that can be good or bad for our bodies.”
Because membranes are made of bilayers – one lipid on one side and another lipid on the other – they are very small, on the order of a couple nanometers, White-Mathieu said, so super-resolution imaging techniques are needed to view the details of these structures.
Expansion microscopy, used to look at proteins, DNA and sugars, hasn’t worked well for lipids, Baskin said. The dynamic nature of membrane formations and processes, sometimes necessary to move the cell’s contents from one side of the membrane to the other, make them difficult to image, especially if you want to image the membranes that are forming those contacts.
“The problem we’re trying to solve with this method is being able to visualize when different parts of the cell have to nestle up close to one another,” he said.
In addition, the hydrophobic nature that makes a lipid a good barrier component also makes it tricky to image with expansion microscopy. “The tagging methods used for this type of microscopy don’t work for lipids, Baskin said. “Brittany’s brilliant idea is in the tagging chemistry.”
To expand an object and not distort it during expansion microscopy, you have to break the links between the components in the object, which normally requires experimental conditions that lose all the lipids, Baskin said. White-Mathieu developed a method that retains the lipids so they can be imaged.
During expansion microscopy, researchers use chemicals to make a polymer that has charged pieces in it, called side chains, White-Mathieu said. When you put the sample in water, the side chains absorb water and expand through osmosis to make the sample bigger.
To apply this general principle to lipid expansion microscopy, White-Mathieu said, she used metabolic labels that have clickable labels on them – that is, an extremely targeted chemical reaction that enables two molecules to snap together quickly and efficiently, like two pieces of a jigsaw puzzle, among thousands, that fit together.
Once the clickable tags are introduced, “the cells will happily take these molecules that have these functional groups on them and incorporate them into lipids,” White-Mathieu said. “From there we take a fluorescent reagent we designed that can react with the clickable handles on lipids. The reagent also contains a polymerizable unit that will get incorporated into the polymer network during the expansion procedure so when the sample is expanded, the lipid is attached directly to the polymer network through the fluorescent compound.”
LExM can potentially be useful in biomedical research, Baskin said, including to study genetic diseases associated with perturbed lipid metabolism, where a lot of fundamental studies of cell metabolism and organization are needed to inform the development of future therapies.
“I think it’s fortuitous timing that Brittany’s paper was published the same week as the Nobel Prize recognizing click chemistry,” Baskin said. “It’s one of hundreds or thousands of illustrations of the power of click chemistry.”
This research was supported by the National Institutes of Health, the National Science Foundation, the Arnold and Mabel Beckman Foundation, the Alfred P. Sloan Foundation and the Howard Hughes Medical Institute.
Kate Blackwood is a writer for the College of Arts and Sciences.
Media Contact
Get Cornell news delivered right to your inbox.
Subscribe