Under-the-skin implant could treat Type I diabetes
By David Nutt, Cornell Chronicle
A collaboration between researchers from Cornell and University of Alberta, Edmonton, has created a new technique to treat Type 1 diabetes: implanting a device inside a pocket under the skin that can secrete insulin while avoiding the immunosuppression that typically stymies management of the disease.
The approach would offer an easier, long-term and less invasive alternative to insulin injections or traditional transplants that require immunosuppression.
The group’s paper, “Inflammation-Induced Neovascularization of the Subcutaneous Tissue for the Long-Term Survival of Encapsulated Islets Without Immunosuppression,” published Dec. 5 in Nature Biomedical Engineering. The co-lead authors are former postdoctoral researcher Long-Hai Wang and Braulio A. Marfil-Garza of University of Alberta, Edmonton.
In Type 1 diabetes, the body’s immune system goes rogue and destroys insulin-producing pancreatic cell clusters, known as islets, thereby depriving the body of a way to usher glucose, i.e., sugar, into muscle and tissue cells to generate energy. The standard treatment for the disease is insulin therapy in the form of daily injections or insulin pumps.
For the last decade, Minglin Ma, professor of biological and environmental engineering in the College of Agriculture and Life Sciences (CALS), has been trying to develop a better way to control the disease.
“Over the years, I receive a lot of emails and requests from parents and patients saying, ‘Hey, my baby was diagnosed with Type 1, can you help us?’’’ Ma said. “It’s a very bad disease, and a lot of children have it. So we are really serious about pushing this into something clinically applicable, something that’s impactful.”
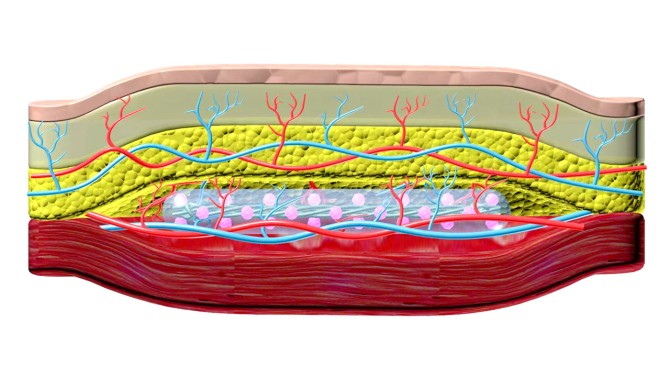
In 2017, he unveiled a removeable polymer thread containing thousands of islet cells, protected by a thin hydrogel coating, that could be implanted in a patient’s abdomen. The device – dubbed TRAFFIC (Thread-Reinforced Alginate Fiber For Islets enCapsulation) – was structured like a tiny micro-porous cage. The enclosed islets could secrete insulin in response to the body’s rising blood-sugar levels while also receiving a steady flow of nutrients and oxygen to stay healthy.
Ma’s lab created a more robust version in 2021 that proved effective in controlling blood sugar in diabetic mice for up to six months.
Those projects led James Shapiro of University of Alberta, Edmonton – “a true leader in islet transplantation,” according to Ma – to reach out about a possible collaboration.
Shapiro had created a method for inserting islets in channels just under a person’s skin, then applying immunosuppression to protect them.
“I was intrigued by the virtue of Ma’s approach as it avoided the need for immunosuppression, and I wondered if we might combine our two innovative strategies to improve cell survival,” Shapiro said. “Indeed it worked.”
The resulting new system is named SHEATH (Subcutaneous Host-Enabled Alginate THread).
The installation is a two-step process. First, a series of nylon catheters are inserted under the skin, where they remain for four to six weeks – long enough for blood vessels to form around the catheters. When the catheters are removed, the islet devices, which are approximately 10 centimeters long, are inserted into the pocket of space the catheters created, and the surrounding vascular system remains intact.
“That channel is a perfect fit for our device. Shapiro used the analogy that this is like a hand in a glove,” Ma said. “And putting something under your skin is much easier, much less invasive than in the abdomen. It can be done as an outpatient procedure, so you don’t have to stay in the hospital. It can be done under local anesthesia.”
The researchers worked with co-authors Ashim Datta, professor of biological and environmental engineering (CALS), whose modeling helped the team understand how the islets in the device can receive nutrients and oxygen, and Dr. James Flanders, associate professor emeritus in the College of Veterinary Medicine, who tested the procedure in pig models.
While additional challenges for the long-term clinical application of the device remain, Ma is hopeful that future versions will be able to last for two to five years before needing to be replaced.
“The challenge is, it’s very difficult to keep these islets functional for a long time inside of the body where you have a device, because the device blocks the blood vessels, but the native islet cells in the body are known to be in direct contact with vessels that provide nutrients and oxygen,” Ma said. “The device is designed in a way that we can maximize the mass exchange of nutrients and oxygen, but we may need to provide additional means to support the cells for a long-term function in large-animal models and eventually patients.”
Ma has formed a new Cornell spinoff, Persista Bio, with his collaborator Linda Tempelman, Ph.D. ’93 serving as CEO, to develop a separate device that can supply additional oxygen to the cells.
Co-authors include doctoral student Alexander Ernst; Kento Okada, MEng ’22; and researchers from University of Alberta, Edmonton, O2M Technologies and University of Science and Technology of China, Hefei.
The research was supported, in part, by the National Institutes of Health, the Novo Nordisk Company, the Juvenile Diabetes Research Foundation, the Hartwell Foundation and the Diabetes Research Institute Foundation of Canada.
Media Contact
Get Cornell news delivered right to your inbox.
Subscribe